filmov
tv
The Third Law of Thermodynamics: Absolute Zero
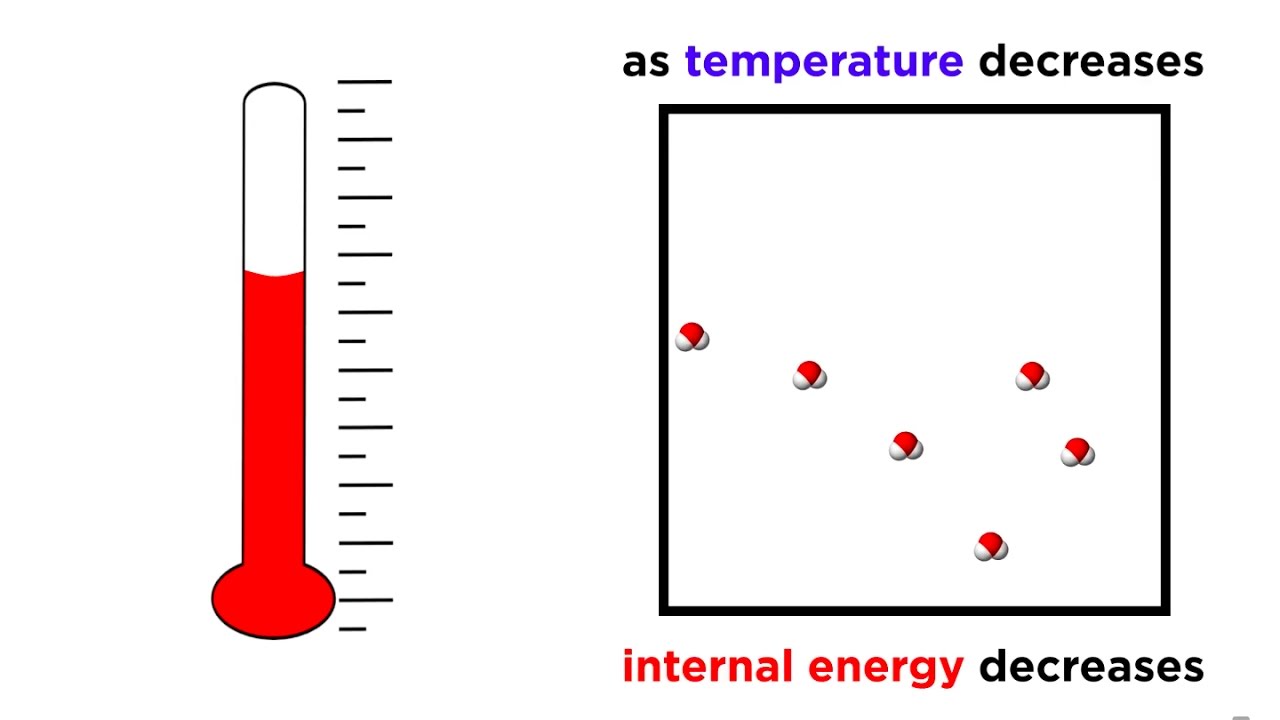
Показать описание
Brr, it's so cold today! Could it get any colder? Is there a coldest possible temperature? Yes, there is! That seems strange, but now we know that temperature is just a measure of kinetic energy, so zero kinetic energy must mean zero temperature! Let's learn more about this winter wonderland of physics as we wrap up our adventures with the laws of thermodynamics.
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
The Third Law of Thermodynamics: Absolute Zero
What is the Third Law of Thermodynamics?
What is the 3rd Law of Thermodynamics? The Third Law Explained!
THIRD LAW OF THERMODYNAMICS | Simple & Basic Animation
The Third Law of Thermodynamics | Physical Chemistry I | 045
The Third Law of Thermodynamics | Science Foundations
Third (3rd) law of Thermodynamics - Concept and Examples
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Master Physics for NUST NET in 1 Day
Chemical Thermodynamics 5.3 - Third Law
Third Law of Thermodynamics
Third law of Thermodynamics
The Second and Third Laws of Thermodynamics
A Mercury Hammer and the Third Law of Thermodynamics
First Law, Second Law, Third Law, Zeroth Law of Thermodynamics
Second and Third Law of Thermodynamics
Second Law of Thermodynamics - Heat Energy, Entropy & Spontaneous Processes
Zeroth, First, Second and Third Laws of Thermodynamics
Understanding the Laws of Thermodynamics: Explaining the Third Law
The Third Law of Thermodynamics
The Zeroth Law of Thermodynamics: Thermal Equilibrium
18.1 The Laws of Thermodynamics | General Chemistry
Lecture 18: Third law of thermodynamics
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
Комментарии
 0:03:41
0:03:41
 0:03:17
0:03:17
 0:08:11
0:08:11
 0:04:03
0:04:03
 0:11:22
0:11:22
 0:00:57
0:00:57
 0:03:24
0:03:24
 0:08:12
0:08:12
 2:06:48
2:06:48
 0:05:01
0:05:01
 0:04:52
0:04:52
 0:02:42
0:02:42
 0:23:03
0:23:03
 0:03:32
0:03:32
 0:01:53
0:01:53
 0:28:02
0:28:02
 0:04:11
0:04:11
 0:06:09
0:06:09
 0:00:59
0:00:59
 0:09:20
0:09:20
 0:03:29
0:03:29
 0:10:06
0:10:06
 0:31:49
0:31:49
 0:11:27
0:11:27