filmov
tv
What is the Third Law of Thermodynamics?

Показать описание
Valeska Ting explains the relationship between entropy, temperature and absolute zero.
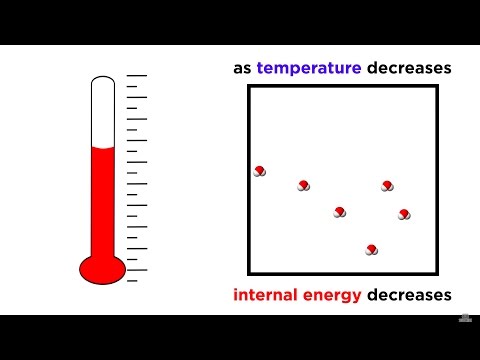
Valeska Ting completes her series of films explaining the four laws of thermodynamics. The third law states that entropy decreases to zero as temperature approaches absolute zero. But what does this mean?
Valeska tackles the theoretical concept of absolute zero, explains the nature of a perfect crystal, and uses a fire extinguisher to illustrate the relationship between entropy and temperature.
Valeska Ting completes her series of films explaining the four laws of thermodynamics. The third law states that entropy decreases to zero as temperature approaches absolute zero. But what does this mean?
Valeska tackles the theoretical concept of absolute zero, explains the nature of a perfect crystal, and uses a fire extinguisher to illustrate the relationship between entropy and temperature.
Newton's Third Law of Motion: Action and Reaction
Newton's 3 Laws, with a bicycle - Joshua Manley
Newton's 3rd Law of Motion | Action and Reaction Forces with Examples | Physics Laws | Dr. Bino...
GCSE Physics - Newton’s Third Law #57
Action and Reaction: Newton’s Third Law (updated)
What is the Third Law of Thermodynamics?
Newton's Third Law | Forces & Motion | Physics | FuseSchool
Newton's Third Law of Motion | Forces and Motion | Physics | Infinity Learn
2025 State Legislative Session: Hunger crisis
Newton’s Third Law of Motion Demonstrated in Space
Newton's Third Law of Motion - Action and Reaction Forces
STEMonstrations: Newton's Third Law of Motion
Best Film on Newton's Third Law. Ever.
Newton's Third Law
Walter Lewin explains Newton's third law
The Third Law of Thermodynamics: Absolute Zero
Newton's Third Law of Motion | Newton's Laws of Motion | Video for Kids
Classroom Experiments- Demonstrating Newton's third law
Newton's 3rd Law - Why does a swimmer push the water backward? | #aumsum #kids #science
Newton's 3rd Law Explained with Skateboard, Rocket
Newton's third law - Best Demonstration EVER !! - by Prof. Walter Lewin
Newton's third law | Physics | Khan Academy
Newton's Third Law
Newtons Third Law of Motion | Action & Reaction
Комментарии
 0:04:55
0:04:55
 0:03:33
0:03:33
 0:05:28
0:05:28
 0:02:36
0:02:36
 0:02:15
0:02:15
 0:03:17
0:03:17
 0:04:48
0:04:48
 0:04:09
0:04:09
 0:00:45
0:00:45
 0:03:08
0:03:08
 0:11:08
0:11:08
 0:03:08
0:03:08
 0:04:38
0:04:38
 0:06:01
0:06:01
 0:00:47
0:00:47
 0:03:41
0:03:41
 0:04:26
0:04:26
 0:00:07
0:00:07
 0:01:40
0:01:40
 0:04:04
0:04:04
 0:00:52
0:00:52
 0:10:27
0:10:27
 0:05:23
0:05:23
 0:00:28
0:00:28