filmov
tv
How a hydrogen atom works | Particle physicist Harry Cliff

Показать описание
#particlephysics #physics #atom #quantumphysics
How a hydrogen atom works | Particle physicist Harry Cliff
Bohr Model of the Hydrogen Atom
Nuclear Bomb: How it Works in detail. Atomic vs Hydrogen bomb (H-bomb)
How Hydrogen Bomb Tsar Bomba Works
A Better Way To Picture Atoms
Hydrogen atom actually looks like THIS! #amazingfacts #science
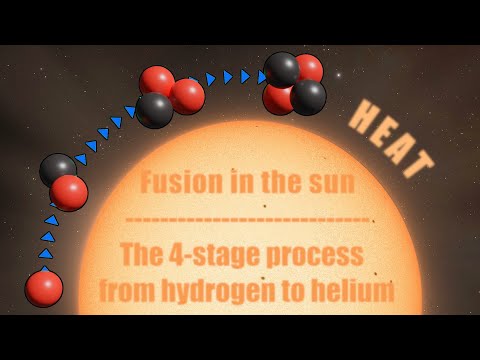
Nuclear fusion in the sun. The 4 steps from hydrogen to helium.
How Do Hydrogen Fuel Cells Work?
Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Seri...
How Does A Hydrogen Car Work | Future Technology
How Small Is An Atom? Spoiler: Very Small.
Nuclear Fusion | Fusion energy explained with Hydrogen atom example | Physics animation video
Energy to Disassemble a Hydrogen Atom
Electrolysis: Producing hydrogen from water
S1.3.1 - The hydrogen emission spectrum
Water into Hydrogen - Making a Simple Hydrogen Generator from old battery#shorts #youtubeshorts
How atoms bond - George Zaidan and Charles Morton
How To Produce Hydrogen And Oxygen From Water/ Electrolysis Of Water, #shorts
Atomic Bomb vs Hydrogen Bomb - How Do They Compare?
how to make a hydrogen cell#youtubeshorts
Atomic Bomb vs. Hydrogen Bomb: Differences Explained
Nuclear Bomb: How it Works in detail. Atomic vs Hydrogen bomb #shorts #trendingshort #geography
A simple battery to split water into hydrogen and oxygen. Chemistry magic or electrolysis #stem
How do Hydrogen-bombs work?
Комментарии
 0:00:55
0:00:55
 0:04:50
0:04:50
 0:09:52
0:09:52
 0:08:42
0:08:42
 0:05:35
0:05:35
 0:00:27
0:00:27
 0:06:10
0:06:10
 0:08:12
0:08:12
 0:21:44
0:21:44
 0:03:33
0:03:33
 0:04:58
0:04:58
 0:03:20
0:03:20
 0:04:47
0:04:47
 0:00:54
0:00:54
 0:08:43
0:08:43
 0:00:58
0:00:58
 0:03:34
0:03:34
 0:00:56
0:00:56
 0:07:53
0:07:53
 0:00:51
0:00:51
 0:00:53
0:00:53
 0:01:00
0:01:00
 0:00:56
0:00:56
 0:00:42
0:00:42