filmov
tv
What's the Difference between Ka and Kb?

Показать описание
Ka is the equilibrium constant for an acid reacting with water to make H3O(+) and its conjugate base.
Kb is the equilibrium constant for a base reacting with water to make OH(-) and its conjugate acid.
The Ka and Kb of a conjugate acid/base pair multiply to give 10^-14.
Kb is the equilibrium constant for a base reacting with water to make OH(-) and its conjugate acid.
The Ka and Kb of a conjugate acid/base pair multiply to give 10^-14.
What's the Difference between Ka and Kb?
Relationship between Ka and Kb | Chemistry | Khan Academy
pKa, Ka, and Acid Strength
Test Your Honey if Naturally Raw or Processed! Dr. Mandell
Ka and Kb
Japanese Method #shorts #fyp
The Difference Between Baking Soda and Baking Powder
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems
How to tell if your avocado is ripe! No more guessing! 🥑🤔🤗 #avocado #foodhacks #veganfood #fyp...
Full form of LOVE | Love ka full form | #shorts #youtubeshorts #viral #love
Sodium metal is soft and squishy
I tested cheap vs expensive footballs!
How To Tell REAL or Fake Diamond (TORCH TEST!)
4 Signs of Ovulation - Ovulation Signs and Symptoms
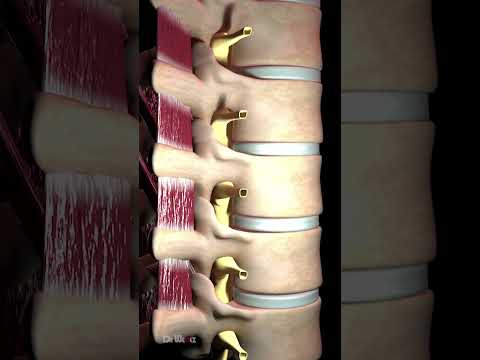
Bulging Disc Explained (Animation)
Ch#8 |Lec#6|Relationship b/w Ka, Kb, Kw#relationship#b/w
X, K, & Ka band: Understanding Radar Detectors
What are X, K, and Ka Band? Five Minute Fridays, Ep. 7
3 Different Types of Dragon Fruit
Drill Bits #shorts
How to Tell If a Diamond is REAL or FAKE (SCRATCH TEST!)
How Heavy Is The Shot Put? #trackandfield
MTB Suspension Comparison:
3 Days Late but Cramping - PMS or Pregnant?
Комментарии
 0:05:37
0:05:37
 0:11:20
0:11:20
 0:05:49
0:05:49
 0:00:46
0:00:46
 0:04:06
0:04:06
 0:00:20
0:00:20
 0:00:28
0:00:28
 0:13:50
0:13:50
 0:00:22
0:00:22
 0:00:25
0:00:25
 0:00:38
0:00:38
 0:00:18
0:00:18
 0:00:23
0:00:23
 0:00:18
0:00:18
 0:00:28
0:00:28
 0:13:18
0:13:18
 0:10:33
0:10:33
 0:06:16
0:06:16
 0:00:27
0:00:27
 0:00:31
0:00:31
 0:00:21
0:00:21
 0:00:15
0:00:15
 0:00:16
0:00:16
 0:00:15
0:00:15