filmov
tv
Alkenes and Alkynes - Naming + Properties

Показать описание
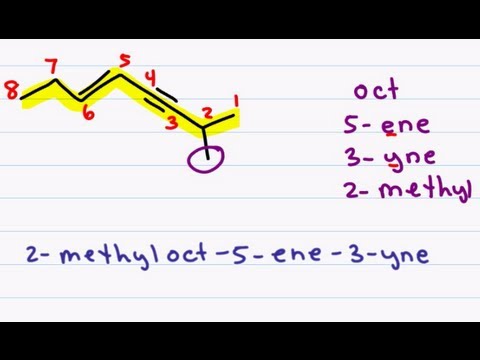
Alkenes are hydrocarbons with DOUBLE bonds. Alkynes are hydrocarbons with TRIPLE bonds.
They are named exactly the same as alkanes, but with ENE and YNE endings, and you might have to give a number to show where the double/triple bond startings in the chain.
They are named exactly the same as alkanes, but with ENE and YNE endings, and you might have to give a number to show where the double/triple bond startings in the chain.
IUPAC Nomenclature of Alkenes and Alkynes
Alkenes and Alkynes - Naming + Properties
IUPAC Nomenclature of Alkenes and Alkynes
Naming alkenes examples | Alkenes and Alkynes | Organic chemistry | Khan Academy
Naming Alkenes and Alkynes on the same Compound - ENYNE *Error at 3:18*
Naming Alkynes - IUPAC Nomenclature & Common Names
Alkenes & Alkynes: Crash Course Chemistry #41
Nomenclature of hydrocarbons: Alkanes, Alkenes, & Alkynes | Chemistry | Khan Academy
How to Write and Name Hydrocarbons | Step-by-Step Guide | ALKANANES | ALKENES | ALKYNES |
naming alkenes and alkynes
Naming Alkenes and Alkynes
Naming alkanes, alkenes and alkynes in organic chemistry
ALEKS: Naming alkenes and alkynes
IUPAC Nomenclature : Alkanes, Alkenes, Alkynes
Naming Alkenes and Alkynes
Naming Alkenes - Naming Alkynes(HD)
12a: Naming alkenes and alkynes
IUPAC Nomenclature of Alkanes - Naming Organic Compounds
Naming Alkenes and Alkynes | Organic Chemistry Nomenclature
Examples for naming alkanes, alkenes and alkynes
Naming Organic Molecules Grade 12 | Alkenes, Alkynes, Haloalkanes
Naming Alkenes - Nomenclature Tutorial for Double Bound Organic Compounds
8.0 Naming Alkenes IUPAC | Organic Chemistry
Iupac nomenclature|| Alkene|| Alkyne|| class 10, 11, 12|| organic chemistry
Комментарии
 0:07:44
0:07:44
 0:08:20
0:08:20
 0:12:43
0:12:43
 0:08:27
0:08:27
 0:05:01
0:05:01
 0:13:16
0:13:16
 0:09:36
0:09:36
 0:09:19
0:09:19
 0:26:18
0:26:18
 0:04:15
0:04:15
 0:04:09
0:04:09
 0:04:26
0:04:26
 0:03:00
0:03:00
 0:12:24
0:12:24
 0:06:24
0:06:24
 0:10:39
0:10:39
 0:11:26
0:11:26
 0:11:18
0:11:18
 0:06:23
0:06:23
 0:13:58
0:13:58
 0:09:59
0:09:59
 0:08:04
0:08:04
 0:18:25
0:18:25
 0:16:10
0:16:10