filmov
tv
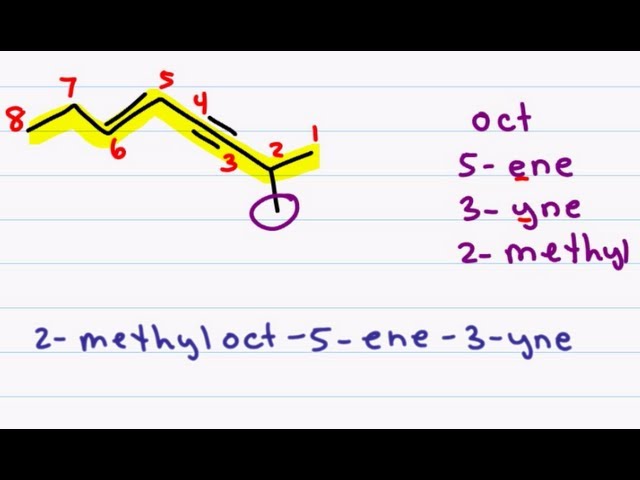
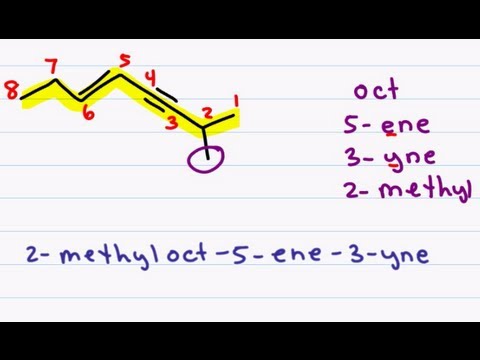
Naming Alkenes and Alkynes on the same Compound - ENYNE *Error at 3:18*
Показать описание
This video is Part 8 in the Naming Organic Compounds series
This nomenclature tutorial video takes you through the IUPAC rules for naming molecules containing both a double and triple bond on the same compound. This is demonstrated using my puzzle piece approach to IUPAC naming with simple and substituted examples
Many students struggle with naming because they attempt to come up with the entire name at once and often wind up missing a piece or two. After this series you won't have that problem anymore
Visit my website for additional naming tutorials:
And of course my ebook: "10 Secrets To Acing Organic Chemistry"
For questions and comments, find me on social media here:
Naming Alkenes and Alkynes on the same Compound - ENYNE *Error at 3:18*
IUPAC Nomenclature of Alkenes and Alkynes
Naming alkenes examples | Alkenes and Alkynes | Organic chemistry | Khan Academy
Naming Alkynes - IUPAC Nomenclature & Common Names
Alkenes & Alkynes: Crash Course Chemistry #41
ALEKS: Naming alkenes and alkynes
Naming Alkenes and Alkynes | Organic Chemistry Nomenclature
Alkenes - how to name them - organic chemistry GCSE
naming alkenes and alkynes
IUPAC Nomenclature of Alkenes and Alkynes
8.0 Naming Alkenes IUPAC | Organic Chemistry
Naming Alkenes - Nomenclature Tutorial for Double Bound Organic Compounds
Naming Alkenes - Naming Alkynes(HD)
Naming Alkenes
Alkenes and Alkynes - Naming + Properties
Nomenclature of hydrocarbons: Alkanes, Alkenes, & Alkynes | Chemistry | Khan Academy
Naming Alkenes and Alkynes
Naming Alkenes Using E Z System - IUPAC Nomenclature
Naming Alkenes and Alkynes
12a: Naming alkenes and alkynes
naming alkenes & alkynes
IUPAC Nomenclature of Alkanes - Naming Organic Compounds
Alkanes | Homologous series | General Organic Chemistry #chemistry #Hydrocarbons #organicchemistry
Step-by-step Writing & Naming Hydrocarbons | ALKANANES | ALKENES | ALKYNES |
Комментарии
 0:05:01
0:05:01
 0:07:44
0:07:44
 0:08:27
0:08:27
 0:13:16
0:13:16
 0:09:36
0:09:36
 0:03:00
0:03:00
 0:06:23
0:06:23
 0:03:16
0:03:16
 0:04:15
0:04:15
 0:12:43
0:12:43
 0:18:25
0:18:25
 0:08:04
0:08:04
 0:10:39
0:10:39
 0:05:15
0:05:15
 0:08:20
0:08:20
 0:09:19
0:09:19
 0:06:24
0:06:24
 0:12:18
0:12:18
 0:04:09
0:04:09
 0:11:26
0:11:26
 0:04:30
0:04:30
 0:11:18
0:11:18
 0:00:16
0:00:16
 0:26:18
0:26:18