filmov
tv
Structure of the Atom | Class 9 Science | Class 9 Science Chapter 4 | Ultra Legend Batch

Показать описание
Thanks For Watching ❤️Like, Subscribe & Share to Appreciate My Hardwork
__________________________________________
Buy Latest book (Affiliate Link)-
*Equipments Used (Affiliate Link)*
________________________________________
FAQ
⦿ When will we start live classes / Facecam Videos?
After We have 300K Subscribers
________________________________________
#class9science
Topics covered in this video -
✔️ Structure of the Atom
✔️ class 9 science chapter 4
✔️ Class 9 Chemistry Chapter 4 | Structure of the Atom - One Shot Full Chapter Revision
✔️ Structure of Atom Class 9 Term 2 Last Week Preparation | Triumph Batch | Science Class 9
✔️ Structure of Atom | Class 9 Science Full Chapter 4 - One Shot | Chemistry | Target 95+
_________________________________________
Warning- All the Content used in this video is my original work. Reposting or Repurposing of This video is not allowed.
__________________________________________
Thanks for providing background -
Комментарии
 0:05:22
0:05:22
 0:02:20
0:02:20
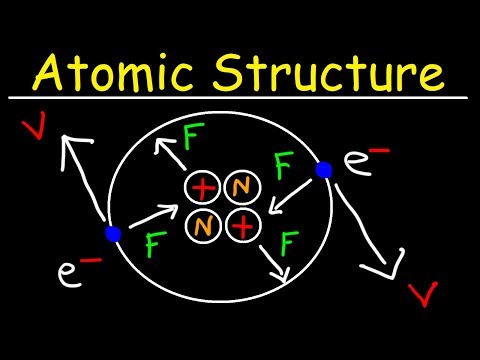 0:11:45
0:11:45
 0:02:02
0:02:02
 0:30:31
0:30:31
 0:01:44
0:01:44
 0:04:00
0:04:00
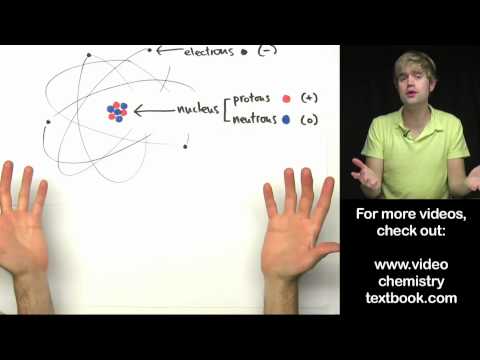 0:07:44
0:07:44
 0:45:43
0:45:43
 0:05:23
0:05:23
 0:09:49
0:09:49
 0:04:06
0:04:06
 0:07:17
0:07:17
 0:33:07
0:33:07
 0:09:42
0:09:42
 0:07:02
0:07:02
 0:13:18
0:13:18
 0:10:43
0:10:43
 0:10:37
0:10:37
 0:02:32
0:02:32
 0:06:34
0:06:34
 0:00:12
0:00:12
 0:04:26
0:04:26
 0:07:20
0:07:20