filmov
tv
Quantum Chemistry 7.7 - Hydrogen Atom Radius (Old Version)

Показать описание
Quantum Chemistry 7.7 - Hydrogen Atom Radius
Quantum Chemistry 7.1 - Hydrogen Atom Model
QUANTUM CHEMISTRY 7 || Wavefunction of Hydrogen atom ||CH1213
Quantum Chemistry 7.7 - Hydrogen Atom Radius (Old Version)
Chapter 7: Bohr Model of Hydrogen | CHM 103 | 082
Chapter 7: Hydrogen Emission Spectrum | CHM 103 | 081
Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Seri...
Hydrogen 7: s-orbitals
Quantum Chemistry 7.5 - Hydrogen Atomic Orbital Nodes
Quantum Chemistry 7
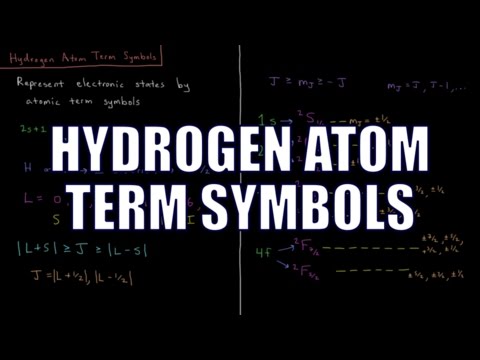
Quantum Chemistry 7.13 - Hydrogen Atom Term Symbols
Quantum Chemistry 7.0 - Hydrogen Atom Review (Old Version)
Understanding Quantum Mechanics #7: Atomic Energy Levels
Bohr Model of the Hydrogen Atom
Carbon Laser Peel treatment at Skinaa Clinic | Viral #shorts
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Quantum Chemistry 7.6 - Hydrogen Atom Eigenvalues
Quantum Chemistry 7.2 - Hydrogen Atom Energies (Old Version)
Quantum Mechanical Model (Part 7 of 9) - Orbital Energies in the Hydrogen Atom (A Special Case)
Lecture 12 (1 of 7) - The Hydrogen Atom
GENERAL CHEMISTRY explained in 19 Minutes
4.2 (Part 7) | The Hydrogen Atom | Introduction to Quantum Mechanics (Griffiths)
Isaac Newton's INSANE Sleep Habits 😬
Quantum Chemistry 7.6 - Hydrogen Atom Eigenvalues (Old Version)
Комментарии
 0:12:39
0:12:39
 0:05:18
0:05:18
 0:41:57
0:41:57
 0:19:26
0:19:26
 0:10:39
0:10:39
 0:07:10
0:07:10
 0:21:44
0:21:44
 0:06:03
0:06:03
 0:07:10
0:07:10
 0:15:01
0:15:01
 0:04:17
0:04:17
 0:08:39
0:08:39
 0:11:20
0:11:20
 0:04:50
0:04:50
 0:00:30
0:00:30
 0:08:42
0:08:42
 0:07:25
0:07:25
 0:10:18
0:10:18
 0:03:18
0:03:18
 0:13:34
0:13:34
 0:18:49
0:18:49
 0:11:03
0:11:03
 0:00:24
0:00:24
 0:10:20
0:10:20