filmov
tv
Understanding Quantum Mechanics #7: Atomic Energy Levels

Показать описание
Correction to what I say at 7 mins 20 seconds: These are the figures for m plus/minus 2, not (as I say) for m plus/minus 1. What's in the figure legend is correct.
The reference I mention at 6 mins 23 seconds is here:
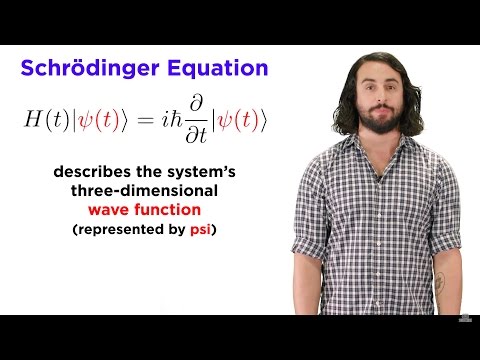
In this video I explain how one calculates energy levels of electrons in orbits around atomic nuclei and why it doesn't really make sense to call them orbits. The images shown are actual solutions of the Schrödinger equation with a Coulomb potential.
#physics #education #science
0:00 Intro and Motivation
1:02 How the Calculation Works
3:05 Properties of the Solutions
7:39 Current Research
10:31 Sponsor Message
Understanding Quantum Mechanics #7: Atomic Energy Levels
Brian Cox explains quantum mechanics in 60 seconds - BBC News
Quantum Physics for 7 Year Olds | Dominic Walliman | TEDxEastVan
Quantum Mechanics Explained in Ridiculously Simple Words
If You Don't Understand Quantum Physics, Try This!
Quantum 101 Episode 1: Wave Particle Duality Explained
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers
Schrödinger's cat: A thought experiment in quantum mechanics - Chad Orzel
24/7 | The Truth Behind the Big Bang! | Space Mysteries To Sleep To |
Quantum Physics for Dummies (A Quick Crash Course!)
Our Universe Has 11 Dimensions, According to Quantum Physics | Billy Carson
A Brief History of Quantum Mechanics - with Sean Carroll
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Quantum Mechanics and the Schrödinger Equation
2021 Live Review 7 | AP Physics 2 | Understanding Quantum, Atomic, and Nuclear Physics
Understanding Quantum Mechanics #6: It's not just a theory for small things
Particles and waves: The central mystery of quantum mechanics - Chad Orzel
Understanding Quantum Mechanics #1: It’s not about discreteness
The Hydrogen Atom, Part 1 of 3: Intro to Quantum Physics
The Schrödinger Equation Explained in 60 Seconds
Quantum Mechanics - Part 1: Crash Course Physics #43
Understanding Quantum Mechanics #4: It's not so difficult!
Quantum Mechanical Model
Quantum Mechanics of the Electron
Комментарии
 0:11:20
0:11:20
 0:01:22
0:01:22
 0:15:36
0:15:36
 0:07:47
0:07:47
 0:12:45
0:12:45
 0:03:32
0:03:32
 0:11:19
0:11:19
 0:04:38
0:04:38
 2:15:16
2:15:16
 0:08:32
0:08:32
 0:09:56
0:09:56
 0:56:11
0:56:11
 0:08:42
0:08:42
 0:06:28
0:06:28
 0:49:08
0:49:08
 0:08:04
0:08:04
 0:04:52
0:04:52
 0:03:07
0:03:07
 0:18:35
0:18:35
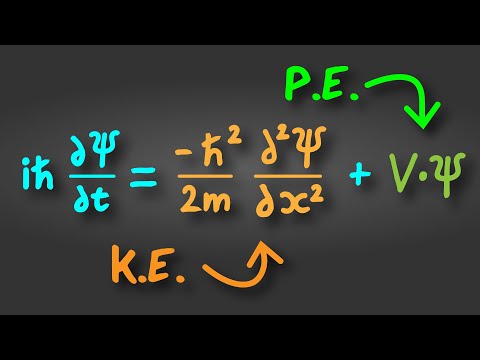 0:01:00
0:01:00
 0:08:45
0:08:45
 0:08:05
0:08:05
 0:04:36
0:04:36
 0:04:01
0:04:01