filmov
tv
Hess's Law Example Problem

Показать описание
A walkthrough for a sample thermochemistry problem involving Hess's Law.
TRANSCRIPT:
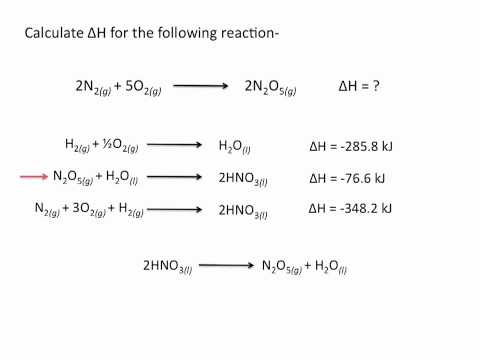
So we need to calculate this enthalpy for this reaction, and we’re given three subreactions to do that. And to complete this, we need to use Hess’s Law, which basically states that the enthalpies of the constituent reactions add up to the enthalpy of the total reaction. So let’s do this.
First we need to manipulate these three equations so that they add up to this. I see here in this first equation that we have a C2H2 on this side, on the left side, but the total reaction requires the C2H2 to be on the right side. So let’s just reverse this whole first equation and that will give us 2 CO2 + H2O yields C2H2 + 5/2 O2. And as you can see, I wrote the delta Hs on the side, and when we reverse the equation, we need to do the same thing to the delta H, or the enthalpy of the reaction. So, instead of a negative 1299.5 kJ/mole enthalpy change, we’re going to have a positive enthalpy change. So this is going to be positive 1299.5 kJ/mole of reaction. And a mole of reaction is just this whole equation right here. Now, let’s go on to the next one; I see that the carbon is in the same place (on the left side of the equation), but we need 2 moles of the carbon. So we have to multiply this whole equation by 2. And what we do over here is going to be the same thing for the enthalpies. So instead of -393.5, we’re going to multiply that by 2, which gives us -787 kJ/mole of reaction. And we now have 2 C + 2O2 yields 2 moles of CO2. Okay, so the last one. I see that we have H2, it’s on the left of the equation like we need it to be over here, we could just ignore these, and ignore. So it looks like we could just leave this third equation by itself, and I also said that we could ignore these because they’re not even in the equation here. So that’s gonna tell us that they’re probably going to cancel out once we add up these three. So let’s see how that works. I forgot to write the enthalpy for the last equation, so let me just write that down. And now let’s add these all together. So I see 2 CO2 over here, is there any other place where we have CO2? And yes, it’s over here. These two cancel out because they’re on opposite sides of the equation. Now I have H2O, I have another H2O over here, and since this one’s on the left, this one’s on the right, they cancel out. C2H2, I don’t see any more, so we’re just going to leave that on the right side of the equation. Then we have 5/2 O2. Now, I see O2 over here and O2 over here. When we add 2 and 1/2 , that’s going to leave us with 5/2. And now we’re left with 2C + H2 yields C2H2, just like we needed over here. So that’s a sure sign that we’re on the right track. Now all we have to do is add up these enthalpies, so 1299.5 – 787 – 285.8 which gives us 226.7 kJ/mole. And that should be the answer.
TRANSCRIPT:
So we need to calculate this enthalpy for this reaction, and we’re given three subreactions to do that. And to complete this, we need to use Hess’s Law, which basically states that the enthalpies of the constituent reactions add up to the enthalpy of the total reaction. So let’s do this.
First we need to manipulate these three equations so that they add up to this. I see here in this first equation that we have a C2H2 on this side, on the left side, but the total reaction requires the C2H2 to be on the right side. So let’s just reverse this whole first equation and that will give us 2 CO2 + H2O yields C2H2 + 5/2 O2. And as you can see, I wrote the delta Hs on the side, and when we reverse the equation, we need to do the same thing to the delta H, or the enthalpy of the reaction. So, instead of a negative 1299.5 kJ/mole enthalpy change, we’re going to have a positive enthalpy change. So this is going to be positive 1299.5 kJ/mole of reaction. And a mole of reaction is just this whole equation right here. Now, let’s go on to the next one; I see that the carbon is in the same place (on the left side of the equation), but we need 2 moles of the carbon. So we have to multiply this whole equation by 2. And what we do over here is going to be the same thing for the enthalpies. So instead of -393.5, we’re going to multiply that by 2, which gives us -787 kJ/mole of reaction. And we now have 2 C + 2O2 yields 2 moles of CO2. Okay, so the last one. I see that we have H2, it’s on the left of the equation like we need it to be over here, we could just ignore these, and ignore. So it looks like we could just leave this third equation by itself, and I also said that we could ignore these because they’re not even in the equation here. So that’s gonna tell us that they’re probably going to cancel out once we add up these three. So let’s see how that works. I forgot to write the enthalpy for the last equation, so let me just write that down. And now let’s add these all together. So I see 2 CO2 over here, is there any other place where we have CO2? And yes, it’s over here. These two cancel out because they’re on opposite sides of the equation. Now I have H2O, I have another H2O over here, and since this one’s on the left, this one’s on the right, they cancel out. C2H2, I don’t see any more, so we’re just going to leave that on the right side of the equation. Then we have 5/2 O2. Now, I see O2 over here and O2 over here. When we add 2 and 1/2 , that’s going to leave us with 5/2. And now we’re left with 2C + H2 yields C2H2, just like we needed over here. So that’s a sure sign that we’re on the right track. Now all we have to do is add up these enthalpies, so 1299.5 – 787 – 285.8 which gives us 226.7 kJ/mole. And that should be the answer.
Комментарии
 0:14:03
0:14:03
 0:04:11
0:04:11
 0:04:58
0:04:58
 0:03:11
0:03:11
 0:06:59
0:06:59
 0:11:23
0:11:23
 0:03:07
0:03:07
 0:13:46
0:13:46
 0:13:10
0:13:10
 0:13:59
0:13:59
 0:12:54
0:12:54
 0:09:34
0:09:34
 0:14:48
0:14:48
 0:07:25
0:07:25
 0:08:49
0:08:49
 0:10:16
0:10:16
 0:06:45
0:06:45
 0:17:41
0:17:41
 0:05:44
0:05:44
 0:02:34
0:02:34
 0:20:52
0:20:52
 0:08:52
0:08:52
 0:00:15
0:00:15
 0:06:16
0:06:16