filmov
tv
12.7 Elimination Reactions of Alcohols | Organic Chemistry

Показать описание
Chad covers the elimination of alcohols using concentrated sulfuric acid (H2SO4). He shows that elimination occurs via the E1 mechanism for tertiary and secondary alcohols and the E2 mechanism for primary alcohols. Chad also compares and contrasts unimolecular vs bimolecular dehydration and the significance of a difference in temperature. Finally, Chad concludes the lesson by showing the Pinacol Rearrangement including the complete mechanism.
00:00 Lesson Introduction
00:46 Elimination of Alcohols with conc. H2SO4 (including mechanisms)
06:55 Unique Elimination of an Alcohol with Rearrangement
10:12 Unimolecular vs Bimolecular Dehydration of Alcohols
13:52 The Pinacol Rearrangement (including mechanism)
00:00 Lesson Introduction
00:46 Elimination of Alcohols with conc. H2SO4 (including mechanisms)
06:55 Unique Elimination of an Alcohol with Rearrangement
10:12 Unimolecular vs Bimolecular Dehydration of Alcohols
13:52 The Pinacol Rearrangement (including mechanism)
12.7 Elimination Reactions of Alcohols | Organic Chemistry
Elimination Reactions of Alcohols from www.ChemistryTuition.Net
E1 and E2 Reactions: Crash Course Organic Chemistry #22
Ch 7 section 12 make Alcohols into leaving groups for substitution and elimination reactions
Elimination Reaction of Alcohols (Class 12 Chemistry)
Lecture Video Ch12 17 Elimination Reactions of Alcohols
12.6 Substitution Reactions of Alcohols | Organic Chemistry
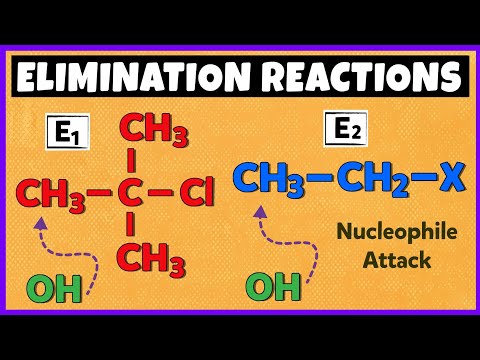
E1 and E2 Elimintaion Reactions | Mechanism
Organic Chemistry - Reaction Mechanisms - Addition, Elimination, Substitution, & Rearrangement
Alcohol dehydration practice
Simplifying Elimination Reactions - Grade 12 Organic Chemistry
Reaction Types Organic chemistry grade 12
7.12 Substitution And Elimination Reactions With Other Substrates PART 1
E1 reactions | Substitution and elimination reactions | Organic chemistry | Khan Academy
Reactions Organic Chemistry Grade 12 | Alkene - Alcohol
Elimination reactions of haloalkanes
Trick to learn 20 Name Reactions in Organic Chemistry | Cass 12
E1 Reaction
18/Elimination Reactions of Alcohols/Hydroxy Compounds and Ethers/Explanation in Tamil
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24
Reactions Organic Chemistry Grade 12 | Alkane to Haloalkane
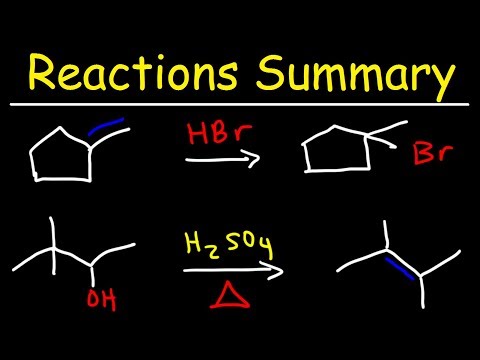
Organic Chemistry Reactions Summary
Year 12 Organic Chemistry Revision - Learning the reactions of Alcohols and Haloalkanes
Elimination Reaction Of Alcohols|Chemistry 12|NEET|JEE|Tamil|Karthikeyan#neetjee#tamil#murugamp
Комментарии
 0:18:56
0:18:56
 0:04:37
0:04:37
 0:13:58
0:13:58
 0:15:21
0:15:21
 0:00:58
0:00:58
 0:01:34
0:01:34
 0:16:34
0:16:34
 0:16:06
0:16:06
 0:34:45
0:34:45
 0:14:06
0:14:06
 0:15:55
0:15:55
 0:03:06
0:03:06
 0:11:27
0:11:27
 0:09:22
0:09:22
 0:07:22
0:07:22
 0:11:04
0:11:04
 0:17:38
0:17:38
 0:02:03
0:02:03
 0:20:55
0:20:55
 0:12:30
0:12:30
 0:03:34
0:03:34
 0:38:34
0:38:34
 0:21:35
0:21:35
 0:20:31
0:20:31