filmov
tv
Elimination reactions of haloalkanes

Показать описание
Hydroxide ions just can't make up their minds, one minute they are nucleophiles the next they are bases! To clear up this confusion watch this video to find out how dissolving hydroxide ions in alcohol makes them undergo elimination reactions to make alkenes ready to use in plastics. Complete with mechanisms too! Woo hoo!!!
Elimination reactions of haloalkanes
E1 and E2 Elimintaion Reactions | Mechanism
Elimination of Halogenoalkanes to form Alkenes (A-Level Chemistry)
substitution & elimination reactions of alkyl halides
HaloAlkanes and HaloArenes 06 : Properties of Haloalkane 3 :Elimination Reaction - E1 & E2 JEE/N...
Halogenoalkanes - Elimination Reactions
SN2 SN1 E1 E2 Reaction Mechanisms Made Easy!
AQA Elimination Reactions in Haloalkanes
🧪 Haloalkanes and haloarenes | Previous year question + NCERT exemplar | NEET 2025 Chemistry
Yr 12 Halogenoalkane elimination reaction
Introduction to elimination reactions | Haloalkanes and Haloarenes | Chemistry | Khan Academy
Elimination reactions of Alkyl halides | E1 and E2 reactions | ch#10 | 12th class chemistry
Class 11th – Reactions of Haloalkanes - Elimination Reactions | Tutorials Point
Halogenoalkanes | A level Chemistry
Elimination Reactions, Reaction With Metals - Haloalkanes and Haloarenes | Class 12 Chemistry Ch 6
AQA A-Level Chemistry - Elimination
Elimination reactions in halogenoalkanes
Ch#17 |Lec#4 | E1 and E2 Reactions and mechanism, Elimination Reactions and types
Tricks to write the products of Elimination Reactions by Komali mam
3. 12C10.4 CV 2 SN 1 Elimination Reaction in Haloalkanes
Haloalkanes & Haloarenes Class 12 #2| Chapter 10 | CBSE NEET JEE - Chemical Reactions , Isomeris...
saytzeff rule #shorts #dehydrohalogenation reaction #elimination reaction #chemistry
Elimination reaction [ saytzeff and hoffmann Products] | Halo Alkanes Halo Arenes | NEET JEE AIIMS
Elimination Reactions of Haloalkanes | Class 12 | NCERT | NEET | in Tamil
Комментарии
 0:11:04
0:11:04
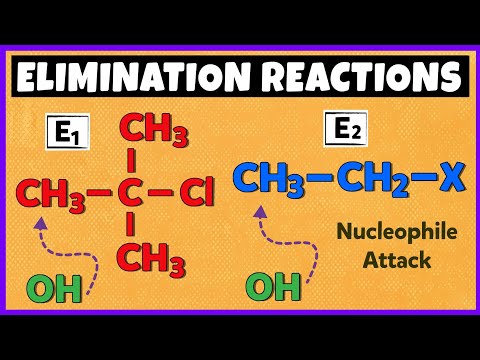 0:16:06
0:16:06
 0:07:57
0:07:57
 0:03:31
0:03:31
 1:46:32
1:46:32
 0:28:15
0:28:15
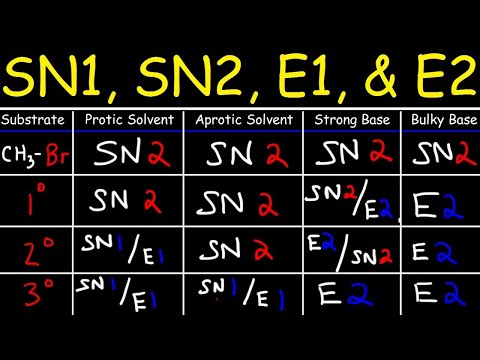 0:38:50
0:38:50
 0:07:08
0:07:08
 1:10:39
1:10:39
 0:03:13
0:03:13
 0:05:42
0:05:42
 0:17:55
0:17:55
 0:08:26
0:08:26
 0:45:12
0:45:12
 1:18:14
1:18:14
 0:12:16
0:12:16
 0:14:57
0:14:57
 0:24:03
0:24:03
 0:34:03
0:34:03
 0:06:50
0:06:50
 2:08:43
2:08:43
 0:00:18
0:00:18
 0:27:42
0:27:42
 0:18:21
0:18:21