filmov
tv
12.6 Substitution Reactions of Alcohols | Organic Chemistry

Показать описание
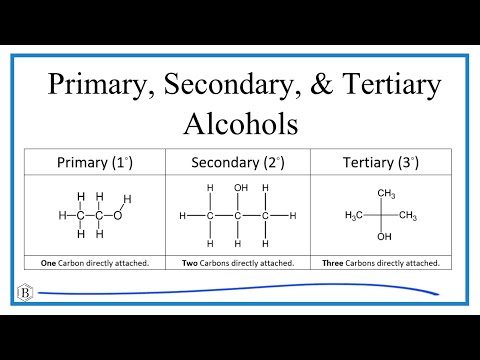
Chad presents a variety of alcohol substitution reactions. The hydroxyl group of an alcohol is a poor leaving group but can be converted to halide or a sulfonate ester both of which are good leaving groups. The reaction of alcohols with the strong acids HI, HBr, and HCl can be used to convert alcohols to the corresponding alkyl halides. For tertiary and secondary alcohols these reactions proceed through the SN1 mechanism, while for primary alcohols and methanol they proceed through the SN2 mechanism (mechanisms shown). PBr3 can also be used to convert primary and secondary alcohols to alkyl bromides via the SN2 mechanism. SOCl2 can also be used to convert primary and secondary alcohols to alkyl chlorides via the SN2 mechanism (the SNi mechanism is mentioned but not covered explicitly). Finally, Chad shows how TsCl (tosyl chloride) can be used to convert alcohols to tosylate esters which are a very good leaving group.
00:00 Lesson Introduction
00:29 Reactions of Alcohols with Hydrogen Halides (HI, HBr, and HCl) with Mechanisms
06:52 Reaction of Alcohols with PBr3 with Mechanism
09:46 Reaction of Alcohols with SOCl2 (Thionyl Chloride) with Mechanism
12:33 Reaction of Alcohols with TsCl (Tosyl Chloride) with Mechanism
00:00 Lesson Introduction
00:29 Reactions of Alcohols with Hydrogen Halides (HI, HBr, and HCl) with Mechanisms
06:52 Reaction of Alcohols with PBr3 with Mechanism
09:46 Reaction of Alcohols with SOCl2 (Thionyl Chloride) with Mechanism
12:33 Reaction of Alcohols with TsCl (Tosyl Chloride) with Mechanism
Комментарии
 0:16:59
0:16:59
 0:08:03
0:08:03
 0:00:32
0:00:32
 0:10:42
0:10:42
 0:11:10
0:11:10
 0:08:45
0:08:45
 0:14:01
0:14:01
 0:12:30
0:12:30
 0:12:19
0:12:19
 0:20:37
0:20:37
 0:34:45
0:34:45
 0:16:48
0:16:48
 0:16:10
0:16:10
 0:26:45
0:26:45
 0:06:49
0:06:49
 0:10:47
0:10:47
 0:17:38
0:17:38
 0:38:34
0:38:34
 0:12:50
0:12:50
 0:11:28
0:11:28
 0:15:04
0:15:04
 0:10:39
0:10:39
 0:03:45
0:03:45
 0:16:53
0:16:53