filmov
tv
Chemical Bonding Introduction: Hydrogen Molecule, Covalent Bond & Noble Gases
Показать описание
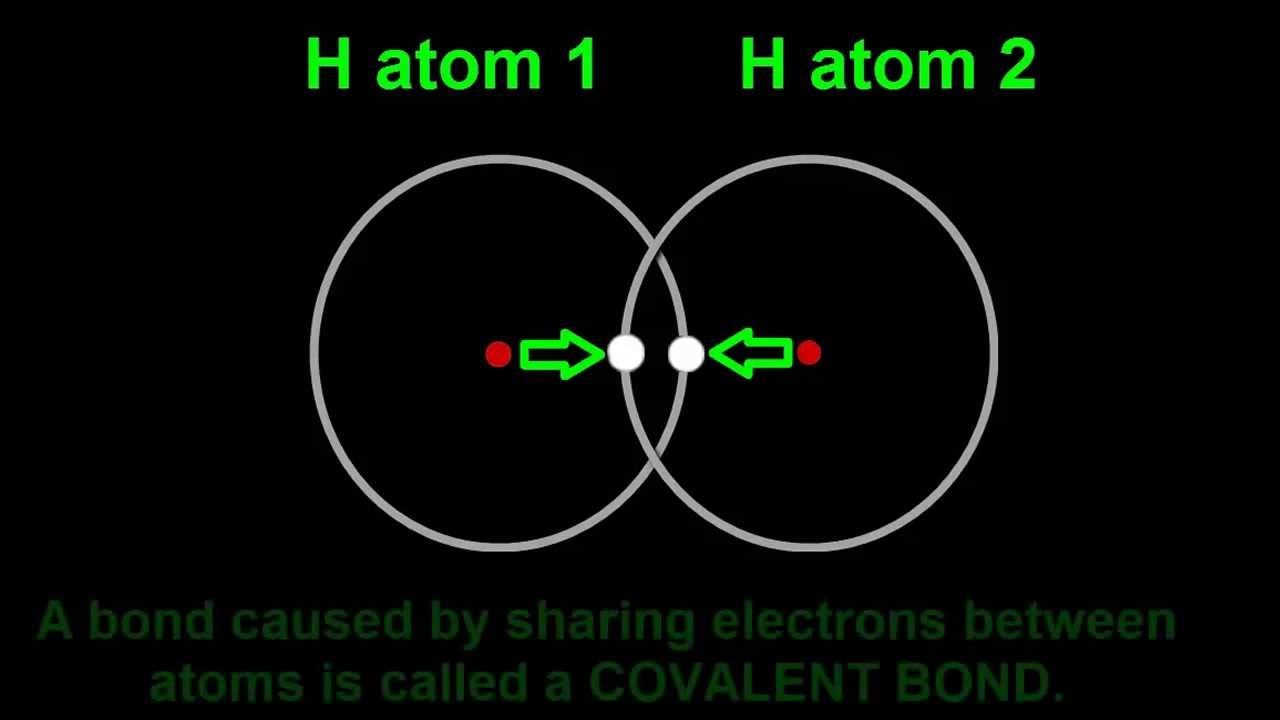
Chemical bonding introduction video shows how covalent bond means 2 hydrogen atoms can stick together to form a hydrogen molecule, H2. The video also explains why helium cannot form bonds and hence is called a noble gas.
Subscribe to watch more online chemistry courses & science videos:
About Atomic School:
Atomic School supports the teaching of Atomic Theory to primary school & science students .
We provide lesson plans, hands-on classroom resources, demonstration equipment, quizzes and a Teacher's Manual to primary school teachers. Animated videos that clearly explain the scientific ideas supports learning by both teachers and students. As a teacher, you don't have to look anywhere else to implement this program.
Our work has been verified by science education researchers at the University of Southern Queensland, Dr Jenny Donovan and Dr Carole Haeusler, who confirm that primary students are capable of learning much more complex scientific concepts than previously thought, and crucially, that they love it. Students run to class!
The program has been trialed in Australian schools as well as schools in the Philippines, Iran and India. It is conducted as holiday workshops at the Australian Nuclear Science and Technology Organisation, the Queensland Museum as well as the World Science Festival.
It has attracted wide media interest, including TV, radio and print, and the research data has been presented at prestigious American Education Research Association and Australian Science Education Research Association conferences.
Atomic Theory underlies all the other sciences- genetics, electronics, nanotechnology, engineering and astronomy- so an early understanding will set them up for a more successful learning sequence for all their science subjects, and support their mastery of mathematics as well. We also have extension programs that cover Biology, Physics and Astronomy to an equal depth.
The founder of Atomic School, Ian Stuart, taught Chemistry and Physics for 25 years at senior levels before he realized that his 8-year old son, Tom, could understand Atomic Theory at a much deeper level than he expected. After visiting Tom's class at school, he discovered that his peers could also grasp the abstract scientific concepts, as well as apply it usefully to the real world.
Ian then developed a program to teach the advanced concepts of high school Chemistry, Physics and Biology to students 10 years younger than they normally would. He found that this engaged their interest in modern science early, and sustained it through to high school and beyond. It also sets them up for future success in their academic and career paths.
Ian has a Bachelor's Degree in Chemistry from the University of Queensland and a Master's degree in Electrochemistry from the University of Melbourne.
Connect with Atomic School on social media:
Video transcript:
Let's do a thought experiment. Imagine a box filled with hydrogen atoms. Like billiard balls on a pool table, atoms actually move, and they do it in straight lines until they hit something … like another hydrogen atom. Oh! See that? They stuck together. They’re not separate hydrogen atoms any more, but a pair of hydrogen atoms moving together. There goes another pair. 4.1 When atoms join up like this, scientists call it a molecule. And they call the join between them a chemical bond.
Here comes another hydrogen atom crashing into the hydrogen molecule. But this time it doesn’t stick. Instead it just bounces off. Hydrogen atoms bond once, and that’s it. They’re just like that. Pretty quickly all the hydrogen atoms will collide and pair off into molecules. They will keep hitting each other, but they'll just bounce off.
Scientists like to have a shorthand way of writing this molecule thingi. Here’s one way to show it, with the hydrogen symbols joined by a stick to show the chemical bond between the atoms. Another way is to write H2, with the little 2 after the H and a bit lower. A number written this way is called a subscript. What do you think the 2 stands for? It counts the number of hydrogen atoms in the molecule. Easy, heh! So when we have a balloon filled with hydrogen gas, it really contains trillions of trillions of H2 molecules.
Let's do another thought experiment. We'll go back to our box filled with hydrogen atoms, but this time put an oxygen atom in there too. When a hydrogen atom crashes into an oxygen atom, they stick together. But wait, when another hydrogen atom hits, it also sticks to the oxygen. What about a third hydrogen atom? No, that’s if for oxygen. It can only make 2 bonds and then it’s done.
Subscribe to watch more online chemistry courses & science videos:
About Atomic School:
Atomic School supports the teaching of Atomic Theory to primary school & science students .
We provide lesson plans, hands-on classroom resources, demonstration equipment, quizzes and a Teacher's Manual to primary school teachers. Animated videos that clearly explain the scientific ideas supports learning by both teachers and students. As a teacher, you don't have to look anywhere else to implement this program.
Our work has been verified by science education researchers at the University of Southern Queensland, Dr Jenny Donovan and Dr Carole Haeusler, who confirm that primary students are capable of learning much more complex scientific concepts than previously thought, and crucially, that they love it. Students run to class!
The program has been trialed in Australian schools as well as schools in the Philippines, Iran and India. It is conducted as holiday workshops at the Australian Nuclear Science and Technology Organisation, the Queensland Museum as well as the World Science Festival.
It has attracted wide media interest, including TV, radio and print, and the research data has been presented at prestigious American Education Research Association and Australian Science Education Research Association conferences.
Atomic Theory underlies all the other sciences- genetics, electronics, nanotechnology, engineering and astronomy- so an early understanding will set them up for a more successful learning sequence for all their science subjects, and support their mastery of mathematics as well. We also have extension programs that cover Biology, Physics and Astronomy to an equal depth.
The founder of Atomic School, Ian Stuart, taught Chemistry and Physics for 25 years at senior levels before he realized that his 8-year old son, Tom, could understand Atomic Theory at a much deeper level than he expected. After visiting Tom's class at school, he discovered that his peers could also grasp the abstract scientific concepts, as well as apply it usefully to the real world.
Ian then developed a program to teach the advanced concepts of high school Chemistry, Physics and Biology to students 10 years younger than they normally would. He found that this engaged their interest in modern science early, and sustained it through to high school and beyond. It also sets them up for future success in their academic and career paths.
Ian has a Bachelor's Degree in Chemistry from the University of Queensland and a Master's degree in Electrochemistry from the University of Melbourne.
Connect with Atomic School on social media:
Video transcript:
Let's do a thought experiment. Imagine a box filled with hydrogen atoms. Like billiard balls on a pool table, atoms actually move, and they do it in straight lines until they hit something … like another hydrogen atom. Oh! See that? They stuck together. They’re not separate hydrogen atoms any more, but a pair of hydrogen atoms moving together. There goes another pair. 4.1 When atoms join up like this, scientists call it a molecule. And they call the join between them a chemical bond.
Here comes another hydrogen atom crashing into the hydrogen molecule. But this time it doesn’t stick. Instead it just bounces off. Hydrogen atoms bond once, and that’s it. They’re just like that. Pretty quickly all the hydrogen atoms will collide and pair off into molecules. They will keep hitting each other, but they'll just bounce off.
Scientists like to have a shorthand way of writing this molecule thingi. Here’s one way to show it, with the hydrogen symbols joined by a stick to show the chemical bond between the atoms. Another way is to write H2, with the little 2 after the H and a bit lower. A number written this way is called a subscript. What do you think the 2 stands for? It counts the number of hydrogen atoms in the molecule. Easy, heh! So when we have a balloon filled with hydrogen gas, it really contains trillions of trillions of H2 molecules.
Let's do another thought experiment. We'll go back to our box filled with hydrogen atoms, but this time put an oxygen atom in there too. When a hydrogen atom crashes into an oxygen atom, they stick together. But wait, when another hydrogen atom hits, it also sticks to the oxygen. What about a third hydrogen atom? No, that’s if for oxygen. It can only make 2 bonds and then it’s done.
Комментарии
 0:07:21
0:07:21
 0:03:34
0:03:34
 0:00:19
0:00:19
 0:08:41
0:08:41
 0:02:48
0:02:48
 0:06:31
0:06:31
 0:06:09
0:06:09
 0:04:30
0:04:30
 2:00:43
2:00:43
 0:11:04
0:11:04
 0:13:25
0:13:25
 0:03:33
0:03:33
 0:09:46
0:09:46
 0:03:25
0:03:25
 0:07:03
0:07:03
 0:05:33
0:05:33
 0:10:54
0:10:54
 0:08:50
0:08:50
 0:03:00
0:03:00
 0:05:30
0:05:30
 0:21:36
0:21:36
 0:47:18
0:47:18
 0:02:15
0:02:15
 0:05:19
0:05:19