filmov
tv
Why do Transition Metals Form Coloured Compounds?

Показать описание
Outlining why and how transition metal ions form coloured compounds. d-orbital splitting and electrons absorbing energy to go from their ground state to an excited state is explained, with examples given. White light and complimentary colours are linked to the colour of compounds.
Recap: 00:31
d-orbital splitting: 02:02
Electrons being excited: 05:29
White light and colour: 07:06
Factors affecting colour of transition metal compounds: 08:07
Summary: 09:23
Thank you for watching - if you found the video useful, please like and subscribe!
Recap: 00:31
d-orbital splitting: 02:02
Electrons being excited: 05:29
White light and colour: 07:06
Factors affecting colour of transition metal compounds: 08:07
Summary: 09:23
Thank you for watching - if you found the video useful, please like and subscribe!
Why do Transition Metals Form Coloured Compounds?
Transition metals and their properties | Matter | Chemistry | FuseSchool
Why Do transition Metals Make Colours, Paper 1 - AQA A Level Chemistry
4.1.5 State that transition elements can form more than one ion IB Chemistry SL
Transition Metals in Ionic Formulas
Why do transition metals form complexes and coloured ions?
Why do transition Metals form complexes?
Electronic Configuration - Transition Metals
PSEB CLASS 12TH CHEMISTRY MISSION 2025 REVISION PAPER 02 SEPTEMBER EXAM 💯🎯
Colour of transition metal ions | the d-block elements | Chemistry | Khan Academy
Why are Transition Metal Complexes Coloured?
13.1/S3.1.8 Why do Transition Metals Have Variable Oxidation States? [HL IB Chemistry]
Transition Metal Coloured Complexes
Complex Ions, Ligands, & Coordination Compounds, Basic Introduction Chemistry
why transition metals form complex compound - class 12
13.2 Colour of complex ions (HL)
Why transition metals form complexes?
Why are complexes coloured? | A-level Chemistry | AQA, OCR, Edexcel
Are transition metals vital?
Introduction to transition metals
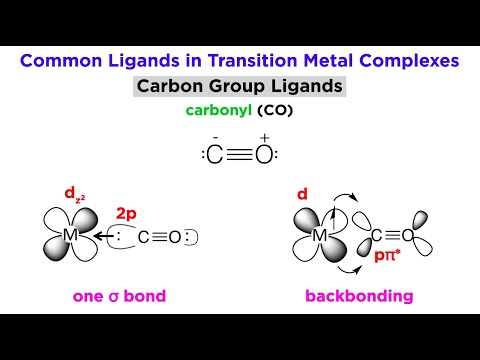
Types of Bonding in Transition Metal Systems and Simple Ligands
27. Introduction to Transition Metals
S3.1.10 Colour of complex ions (HL)
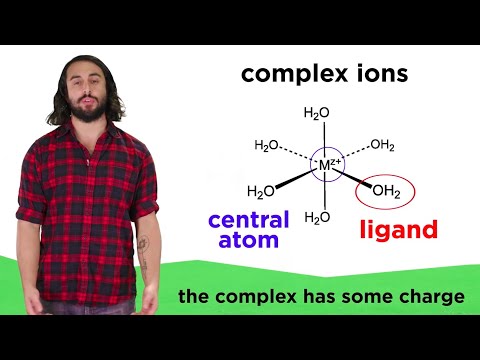
Complex Ion Formation
Комментарии
 0:12:15
0:12:15
 0:03:21
0:03:21
 0:04:16
0:04:16
 0:01:46
0:01:46
 0:08:12
0:08:12
 0:04:17
0:04:17
 0:05:02
0:05:02
 0:04:14
0:04:14
 0:27:20
0:27:20
 0:06:18
0:06:18
 0:07:42
0:07:42
 0:05:04
0:05:04
 0:09:31
0:09:31
 0:13:42
0:13:42
 0:01:20
0:01:20
 0:05:20
0:05:20
 0:02:04
0:02:04
 0:02:42
0:02:42
 0:08:39
0:08:39
 0:11:33
0:11:33
 0:11:54
0:11:54
 0:43:31
0:43:31
 0:07:02
0:07:02
 0:04:06
0:04:06