filmov
tv
4.1.5 State that transition elements can form more than one ion IB Chemistry SL

Показать описание
The reason why this is true is not needed in SL. Specifically mentioned in the syllabus is that iron ions can have a charge of 2+ or 3+. Another example is copper ions with a 1+ or 2+ charge.
4.1.5 State that transition elements can form more than one ion IB Chemistry SL
Electronic Configuration - Transition Metals
S3.1.9 Oxidation states of the transition elements (HL)
Complex Ions, Ligands, & Coordination Compounds, Basic Introduction Chemistry
MDCAT I Transition Elements I Unit 12 I Lec # 1 I Prof. Wajid Ali Kamboh | @wakacademy
Transition Elements lesson 1 of 4 by Dr Bbosa Science
How to Calculate the Oxidation State of Transition Metals in Coordination Compounds
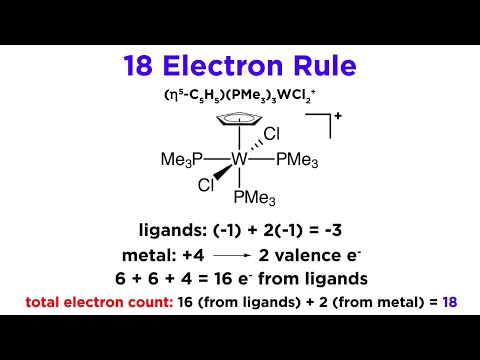
The 18 Electron Rule for Transition Metal Complexes
Refact.ai Match 1 (Codeforces Round 985) Post Contest Discussion by Viram Mehta
Trick To Remember 3d Series, First Transition Elements Series | Periodic Table #InorganicChemistry
Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Seri...
The Groups of the Periodic Table Simplified!
GCSE Chemistry - Group 1 Alkali Metals #11
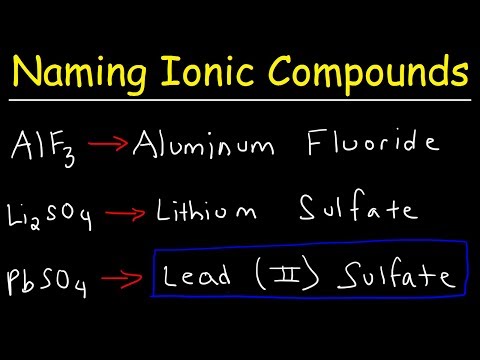
How To Name Ionic Compounds With Transition Metals
Did you know how to remember reactivity series?
5.Transition Elements
Electron Configuration - Basic introduction
finally alakh sir engagement// Babita mil gai ❤️😅 // wife pics / #engaged#alakh sir #fianally
Periodic Table
NEET aspirants then vs now
13.1/S3.1.8 Why do Transition Metals Have Variable Oxidation States? [HL IB Chemistry]
Trying transition video for the first time 💙😂 || #transformation #transition #shorts #viralvideo...
Chemical reactions between metals and water
Transition Elements - Special Characteristics (2020) | SPM & IGCSE
Комментарии
 0:01:46
0:01:46
 0:04:14
0:04:14
 0:04:30
0:04:30
 0:13:42
0:13:42
 0:52:52
0:52:52
 0:22:50
0:22:50
 0:04:58
0:04:58
 0:10:45
0:10:45
 1:03:16
1:03:16
 0:00:45
0:00:45
 0:21:44
0:21:44
 0:04:27
0:04:27
 0:05:46
0:05:46
 0:13:33
0:13:33
 0:00:30
0:00:30
 0:11:09
0:11:09
 0:10:19
0:10:19
 0:00:14
0:00:14
 0:24:09
0:24:09
 0:00:12
0:00:12
 0:05:04
0:05:04
 0:00:15
0:00:15
 0:01:01
0:01:01
 0:12:05
0:12:05