filmov
tv
Structure of Atom | Bohr Model - Energy & Radius Calculations | e-Wave Quantization
Показать описание
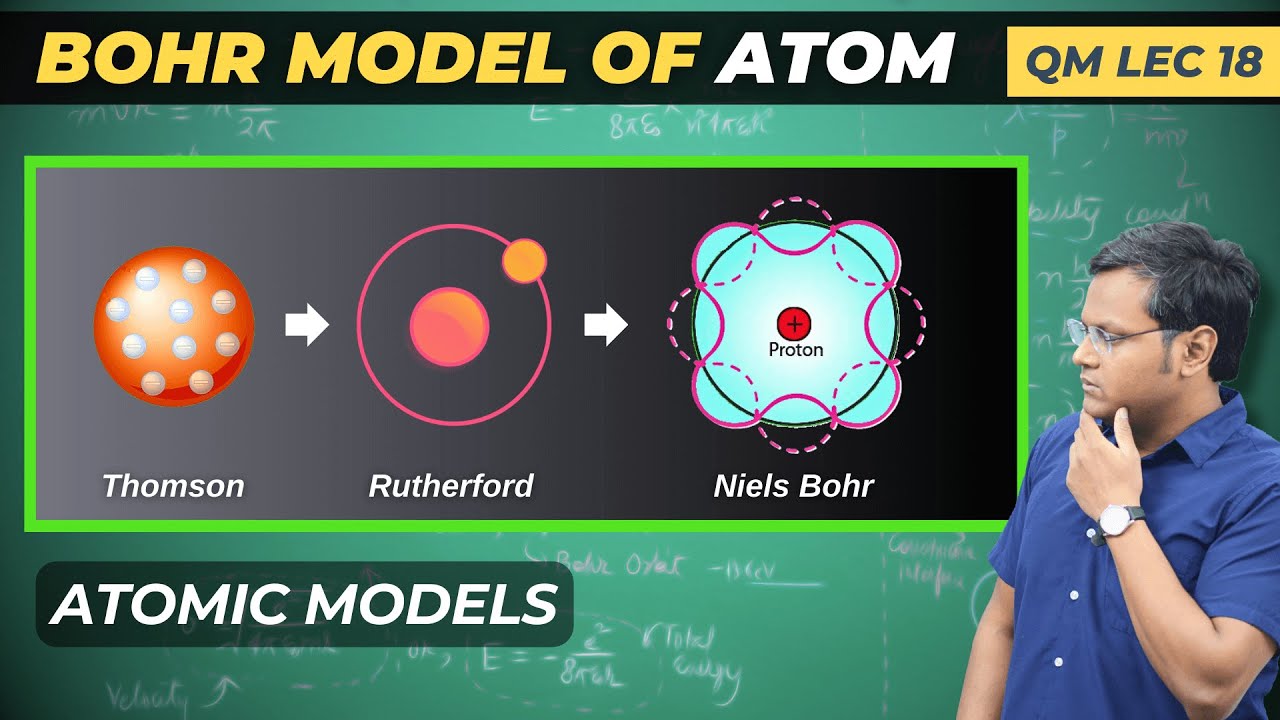
What is the Structure of Atom? Let's understand the various Atomic models and how they came about, starting from Thomson Model, to Rutherford Model, and the Bohr Model of Atom.
𓏬𓏬𓏬𓏬𓏬𓏬𓏬ELEVATE𓏬𓏬𓏬𓏬𓏬𓏬𓏬
*Elevate Classes* - Find LIVE Batches & Recorded Courses for Physics IITJAM, CSIR-NET, GATE, TIFR, JEST etc on our platform
𓏬𓏬𓏬𓏬𓏬𓏬MY NOTES - GDRIVE𓏬𓏬𓏬𓏬𓏬
𓏬𓏬𓏬𓏬VIDEO DETAILS𓏬𓏬𓏬𓏬
What is the Structure of Atom?
In this video, I discuss the very earliest models of the Atom, from JJ Thomson Model, to Rutherford's Planetary Model, to Neils Bohr's Model of the Atom. I also discuss the importance of de-Broglie Hypothesis in explaining the Stability condition for Stationary orbits in the Bohr model. We see how de broglie hypothesis and Electron wave quantisation creates stable orbits in the Bohr model of the Atom and thus provides validity to the dual nature of matter.
00:00 Introduction
01:40 Rutherford Model of Atom
03:50 Energy & Radius Calculations
09:45 Failures of Rutherford Model
14:36 Bohr Model of Atom
22:54 Energy & Radius Calculations
29:46 Electron Wave Quantisation
𓏬𓏬𓏬𓏬𓏬TELEGRAM𓏬𓏬𓏬𓏬
𓏬𓏬𓏬𓏬𓏬SUPPORT𓏬𓏬𓏬𓏬𓏬
Your Financial support provides me an additional incentive to create high quality lecture videos. I am very much thankful for your generosity and kindness
JOIN as a member in Youtube 😇😇😇
𓏬𓏬𓏬𓏬𓏬𓏬𓏬ELEVATE𓏬𓏬𓏬𓏬𓏬𓏬𓏬
*Elevate Classes* - Find LIVE Batches & Recorded Courses for Physics IITJAM, CSIR-NET, GATE, TIFR, JEST etc on our platform
𓏬𓏬𓏬𓏬𓏬𓏬MY NOTES - GDRIVE𓏬𓏬𓏬𓏬𓏬
𓏬𓏬𓏬𓏬VIDEO DETAILS𓏬𓏬𓏬𓏬
What is the Structure of Atom?
In this video, I discuss the very earliest models of the Atom, from JJ Thomson Model, to Rutherford's Planetary Model, to Neils Bohr's Model of the Atom. I also discuss the importance of de-Broglie Hypothesis in explaining the Stability condition for Stationary orbits in the Bohr model. We see how de broglie hypothesis and Electron wave quantisation creates stable orbits in the Bohr model of the Atom and thus provides validity to the dual nature of matter.
00:00 Introduction
01:40 Rutherford Model of Atom
03:50 Energy & Radius Calculations
09:45 Failures of Rutherford Model
14:36 Bohr Model of Atom
22:54 Energy & Radius Calculations
29:46 Electron Wave Quantisation
𓏬𓏬𓏬𓏬𓏬TELEGRAM𓏬𓏬𓏬𓏬
𓏬𓏬𓏬𓏬𓏬SUPPORT𓏬𓏬𓏬𓏬𓏬
Your Financial support provides me an additional incentive to create high quality lecture videos. I am very much thankful for your generosity and kindness
JOIN as a member in Youtube 😇😇😇
Комментарии
 0:01:59
0:01:59
 0:05:05
0:05:05
 0:04:50
0:04:50
 0:06:21
0:06:21
 0:21:44
0:21:44
 0:07:04
0:07:04
 1:12:19
1:12:19
 0:04:38
0:04:38
 1:04:37
1:04:37
 0:02:03
0:02:03
 0:04:06
0:04:06
 0:06:48
0:06:48
 0:02:10
0:02:10
 0:07:00
0:07:00
 0:09:42
0:09:42
 0:01:59
0:01:59
 0:11:45
0:11:45
 0:10:01
0:10:01
 1:08:09
1:08:09
 0:38:12
0:38:12
 0:01:51
0:01:51
 0:21:11
0:21:11
 0:03:47
0:03:47
 0:11:52
0:11:52