filmov
tv
Bohr's Model of an Atom - Class 9 Tutorial

Показать описание
In 1913 Bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. The motion of the electrons in the Rutherford model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curved path emits electromagnetic radiation; thus, the electrons would lose energy and spiral into the nucleus. To remedy the stability problem, Bohr modified the Rutherford model by requiring that the electrons move in orbits of fixed size and energy.
The energy of an electron depends on the size of the orbit and is lower for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump.
The energy of an electron depends on the size of the orbit and is lower for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump.
Bohr Model of the Hydrogen Atom
Bohr Model of an Atom
Bohr's Model of an Atom - Class 9 Tutorial
Bohr’s Atomic Model | Atoms and Molecules | Infinity Learn NEET
The Bohr Atom
What is the Bohr model of the atom?
Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Seri...
How to Draw a Bohr Diagram | Chemistry Homework in 2 MINUTES
Structure of Atom Class 11 | Most Important Concepts & Numericals | CBSE Board Exam & JEE/NE...
What Are The Different Atomic Models? Dalton, Rutherford, Bohr and Heisenberg Models Explained
Bohr's model of an atom | Structure of an atom | Chemistry | Khan Academy
Class 11 chap 2 | Atomic Structure 02 | Bohr's Atomic ModeL | Most Important For IIT JEE and NE...
Bohrs atommodel
Atomic Structure scam hai 🤬🤬 #jee2025 #iit #motivation #iitjee
Bohr's Atomic Model | Chemistry
Structure of an atom| Science project #shorts #projectideas #scienceproject
Atomic Structure (Bohr Model) for Oxygen (O)
#uptgt_science | Bohr atomic model limitations (H-model) | part-2
Bohr's Atomic Model
How small are atoms?
Bohr's atomic Model || Main postulates || 11th class chemistry || ch.no.5
Bohr's Model Of An Atom|| Animated explanation in Hinglish || Atom and Nuclei || Physics 12th c...
Rutherford experiment
#uptgt_science | Bohr atomic model limitations (H-model) | part-1
Комментарии
 0:04:50
0:04:50
 0:02:50
0:02:50
 0:04:06
0:04:06
 0:05:05
0:05:05
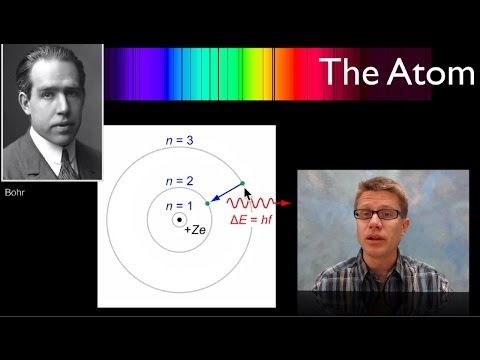 0:06:21
0:06:21
 0:27:12
0:27:12
 0:21:44
0:21:44
 0:01:52
0:01:52
 0:17:06
0:17:06
 0:07:04
0:07:04
 0:02:10
0:02:10
 1:08:09
1:08:09
 0:10:00
0:10:00
 0:00:22
0:00:22
 0:21:11
0:21:11
 0:00:11
0:00:11
 0:01:59
0:01:59
 0:00:05
0:00:05
 1:12:19
1:12:19
 0:00:48
0:00:48
 0:17:38
0:17:38
 0:03:47
0:03:47
 0:00:16
0:00:16
 0:00:05
0:00:05