filmov
tv
Understanding Bohr's Atom: his postulates and the limitations

Показать описание
In this video, I discuss the postulates of Bohr's atom, how it solves the problem of Rutherford's model and how at the same time explains the lines is spectral analysis, including the Balmer lines.
If you like this video, please press the LIKE and SHARE with your peers. And please add a COMMENT to let me know I have helped you.
Follow me
facebook: @physicshigh
twitter: @physicshigh
#physicshigh #highschoolphysicsexplained
If you like this video, please press the LIKE and SHARE with your peers. And please add a COMMENT to let me know I have helped you.
Follow me
facebook: @physicshigh
twitter: @physicshigh
#physicshigh #highschoolphysicsexplained
Understanding Bohr's Atom: his postulates and the limitations
Bohr Model of the Hydrogen Atom
Bohr’s Atomic Model | Atoms and Molecules | Infinity Learn NEET
The Bohr Atom
Louis de Broglie's explanation of Bohr's atomic model
Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Seri...
What Are The Different Atomic Models? Dalton, Rutherford, Bohr and Heisenberg Models Explained
What is the Bohr model of the atom?
Bohr's Atomic Model | Chemistry
Bohr's Model of an Atom - Class 9 Tutorial
Bohr Model of an Atom
Bohr's Model Explained In ONE Minute!!
11C02 - Atomic Structure - Bohr's Atomic Model and Postulates - Ashwin Sir
Postulates of Bohrs theory | Class 11 Chemistry |Structure of Atom Chapter 2 |Part 1
Bohr's atomic model |⚡3d animation | Class 9, Chemistry |
How small are atoms?
Bohr's Atomic Model
Bohr's Model Of An Atom|| Animated explanation in Hinglish || Atom and Nuclei || Physics 12th c...
Deriving the Bohr Radius of the Atom
👉 Postulates of Bohr's Atomic Model 💯 @didyouknow__2002#education #chemistry #viralvideo #trend...
Limitations of Bohr atom model - Class 12 Physics
Bohr atomic model | Jee | neet | infinity approach physics | cbse
Neil Bohr kafi chote the unke samne😱😱||Ft.Alakh.sir!! #physicswallah #AlakhSirSamvad #shorts
Bohr|Atom|Model|Physics 12|Tamil|MurugaMP
Комментарии
 0:11:52
0:11:52
 0:04:50
0:04:50
 0:05:05
0:05:05
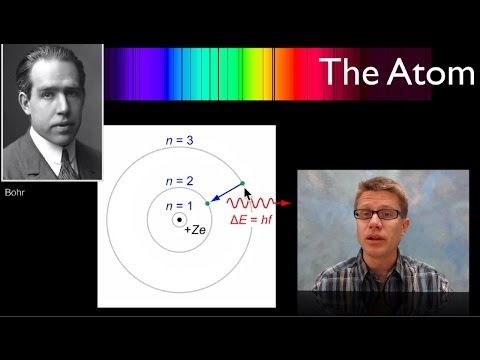 0:06:21
0:06:21
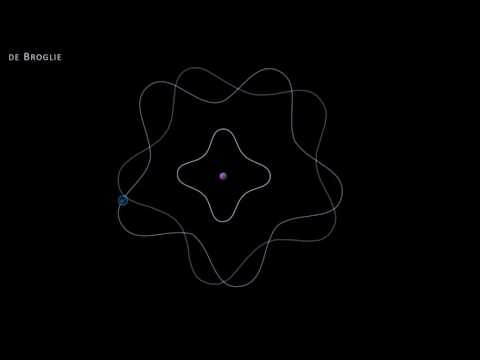 0:08:12
0:08:12
 0:21:44
0:21:44
 0:07:04
0:07:04
 0:27:12
0:27:12
 0:21:11
0:21:11
 0:04:06
0:04:06
 0:02:50
0:02:50
 0:00:59
0:00:59
 0:06:48
0:06:48
 0:07:45
0:07:45
 0:04:38
0:04:38
 0:00:48
0:00:48
 1:12:19
1:12:19
 0:03:47
0:03:47
 0:03:37
0:03:37
 0:00:05
0:00:05
 0:00:11
0:00:11
 0:00:13
0:00:13
 0:00:19
0:00:19
 0:15:30
0:15:30