filmov
tv
Why does heat transfer from hot to cold? (Physics)

Показать описание
Welcome to FactTap! In this video we answer the question: WHY DOES HEAT TRANSFER FROM HOT TO COLD?
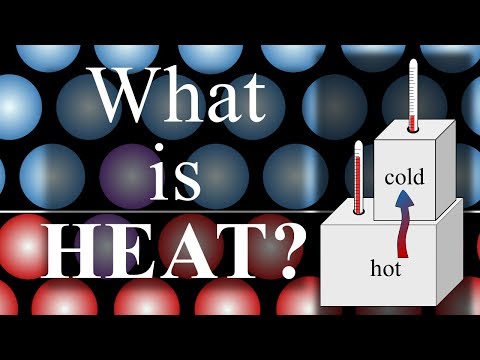
Heat is a form of energy. When energy is added to atoms they begin to move very rapidly. Think of it like kinetic energy. When you push a ball (or add energy to it), it rolls. This means that the more motion atoms have, the hotter they feel to the touch.
Back to the ball example. If you have a stationary ball and you impact it with another ball, the energy of the rolling ball is transferred into the stationary ball, causing it to move. You would say that the stationary ball absorbed the faster balls energy. It wouldn’t be right or sound right to say that the stationary ball transferred its stationary state to the rolling ball. The same is said about atoms transferring energy. The fast moving atoms impact slow moving (cold) atoms, transferring their kinetic energy. Therefore, to say heat transfers from hot to cold really means that energy is transferred from fast moms to slow moving atoms.
If you want to submit questions, leave them in the comments down below and check back next Wednesday for new videos!
Writer & Vocals:
Twitter:
Sources:
Music: n/a
Heat is a form of energy. When energy is added to atoms they begin to move very rapidly. Think of it like kinetic energy. When you push a ball (or add energy to it), it rolls. This means that the more motion atoms have, the hotter they feel to the touch.
Back to the ball example. If you have a stationary ball and you impact it with another ball, the energy of the rolling ball is transferred into the stationary ball, causing it to move. You would say that the stationary ball absorbed the faster balls energy. It wouldn’t be right or sound right to say that the stationary ball transferred its stationary state to the rolling ball. The same is said about atoms transferring energy. The fast moving atoms impact slow moving (cold) atoms, transferring their kinetic energy. Therefore, to say heat transfers from hot to cold really means that energy is transferred from fast moms to slow moving atoms.
If you want to submit questions, leave them in the comments down below and check back next Wednesday for new videos!
Writer & Vocals:
Twitter:
Sources:
Music: n/a
Комментарии
 0:01:05
0:01:05
 0:01:00
0:01:00
 0:03:15
0:03:15
 0:08:36
0:08:36
 0:04:34
0:04:34
 0:11:09
0:11:09
 0:03:16
0:03:16
 0:05:45
0:05:45
 0:35:56
0:35:56
 0:03:04
0:03:04
 0:01:00
0:01:00
 0:01:46
0:01:46
 0:06:57
0:06:57
 0:05:23
0:05:23
 0:17:33
0:17:33
 0:06:56
0:06:56
 0:13:36
0:13:36
 0:02:51
0:02:51
 0:01:37
0:01:37
 0:02:46
0:02:46
 0:06:04
0:06:04
 0:03:23
0:03:23
 0:04:27
0:04:27
 0:09:14
0:09:14