filmov
tv
Calculate for the molarity of the NaOH solution

Показать описание
Question: in a neutralization reaction 45.7 mL of 0.500 M sulfuric acid is required to completely react with 20.0 mL of sodium hydroxide. determine the molarity of the sodium hydroxide solution
B) What is the molarity of a potassium hydroxide solution if 48.0 mL is needed to neutralize 35.0 mL of sulfuric acid?
------------------------
Answered By:
Tiffany W.
Professional Tutor: Math, Science, English, and Standardized Test Prep
------------------------
------------------------
About: Wyzant Ask an Expert offers free answers to your toughest academic and professional questions from over 80,000 verified experts. It’s trusted by millions of students each month with the majority of questions receiving an answer within 1 hour of being asked. If you ever need more than just an answer, Wyzant also offers personalized 1-on-1 sessions with experts that will work with you to help you understand whatever you’re trying to learn.
 0:21:27
0:21:27
 0:04:54
0:04:54
 0:08:46
0:08:46
 0:31:25
0:31:25
 0:01:35
0:01:35
 0:01:41
0:01:41
 0:07:38
0:07:38
 0:09:36
0:09:36
 0:00:38
0:00:38
 0:00:57
0:00:57
 0:05:41
0:05:41
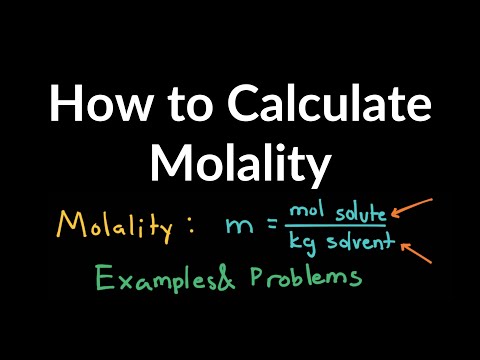 0:05:25
0:05:25
 0:08:21
0:08:21
 0:02:30
0:02:30
 0:03:32
0:03:32
 0:05:05
0:05:05
 0:12:16
0:12:16
 0:04:46
0:04:46
 0:02:54
0:02:54
 0:00:58
0:00:58
 0:15:36
0:15:36
 0:05:23
0:05:23
 0:01:30
0:01:30
 0:04:14
0:04:14