filmov
tv
How to Determine the Most Stable Lewis Structure Practice Problems, Examples, Questions, Summary
Показать описание
👉 Support me on Patreon 👈
💻 My highly recommended chemistry resources
HIGH SCHOOL / GENERAL CHEMISTRY
ORGANIC CHEMISTRY
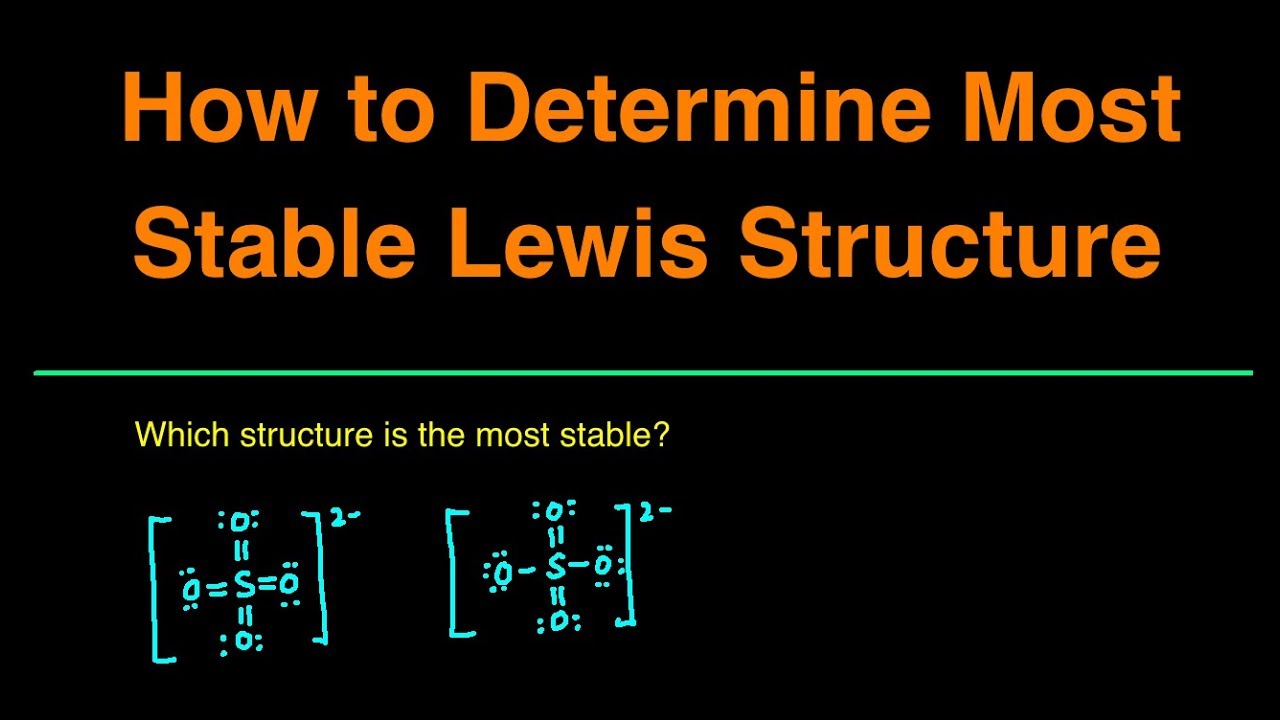
In this video, you'll learn how to determine the most stable or best lewis structure. We'll first start by going over what is formal charge and how to calculate formal charge. Then we'll go over the rules for how to determine the most stable lewis structure.
Then we'll look at three possible structures for sulfate SO4 2- and determine which of the three is the most plausible and most stable lewis structure.
By the end of this video, you'll know exactly how to determine the most stable lewis structure.
How to Determine the Most Stable Lewis Structure Practice Problems, Examples, Questions, Summary
How To Determine Your Core Values | 13 Questions with Dr John Demartini
Periodic Trends - Determine which atom is the most electronegative - Johnny Cantrell
How to determine which isotope is the most abundant
How To Determine Your Core Life Values
How To Determine The Charge of Elements and Ions - Chemistry
How to Determine Testosterone Levels by Looking at Your Ring Finger
How to Determine Which Solid is More Soluble Given Ksp Examples, Shortcut, Problems, Explained
The Most Important Issue of Them All—Abortion (Hank Unplugged Podcast)
How can I determine the reactivity of an element?
Determine Highest pH Solution [Acids and Bases]
Determine the Most Acidic Proton/Molecule
💧💎 The Value Of Things - How Do We Determine It?
How To Determine The Maximum Number of Electrons Using Allowed Quantum Numbers - 8 Cases
How to Determine Your Dominant Eye with Our Dominant Eye Test
How to Determine if Acid is Strong or Weak Shortcut w/ Examples and Practice Problems
'The Most Crucial Step in Judging Someone is to Determine Their Character' Robert Greene
How to determine the rule for a sequence
How To Determine Peoples' Character | Robert Greene on The Laws of Human Nature
How to Determine if Ionic Compound is Soluble or Insoluble in Water Examples, Solubility Rules
Learn how to determine the greatest speed from a velocity graph
Easy Way To Determine Ortho-Para or Meta Directing EAS WITHOUT Memorizing Anything!
How to determine the qiblah when the apps show slightly different angles? - assim al hakeem
How to determine the intervals that a function is increasing decreasing or constant
Комментарии
 0:04:24
0:04:24
 0:12:46
0:12:46
 0:04:10
0:04:10
 0:02:38
0:02:38
 0:38:02
0:38:02
 0:19:12
0:19:12
 0:01:16
0:01:16
 0:05:11
0:05:11
 0:51:48
0:51:48
 0:02:56
0:02:56
 0:01:49
0:01:49
 0:14:10
0:14:10
 0:04:37
0:04:37
 0:11:46
0:11:46
 0:00:33
0:00:33
 0:02:34
0:02:34
 0:09:39
0:09:39
 0:01:49
0:01:49
 0:03:37
0:03:37
 0:04:17
0:04:17
 0:01:52
0:01:52
 0:06:02
0:06:02
 0:03:10
0:03:10
 0:02:56
0:02:56