filmov
tv
How to Determine if Acid is Strong or Weak Shortcut w/ Examples and Practice Problems

Показать описание
👉 Support me on Patreon 👈
💻 My highly recommended chemistry resources
HIGH SCHOOL / GENERAL CHEMISTRY
ORGANIC CHEMISTRY
In this video, you'll learn a super simple technique to determine if a molecule is polar or nonpolar. It is very easy. We first start by discussing what polar and nonpolar means. Then we go through the technique which involves a checklist of two criteria. Lastly we go through 6 examples that are all different to reinforce the technique. By the end, you'll know exactly to easily determine if a molecule is polar or nonpolar.
How to Determine if Acid is Strong or Weak Shortcut w/ Examples and Practice Problems
How to Identify Acid, Base, Conjugate Acid, and Conjugate Base Examples and Practice Problems
Acid Base Strength - Which Is Stronger?
pKa, Ka, and Acid Strength
How to Identify Lewis Acid and Lewis Base Shortcut, Practice Problems, Examples, Explained
Acids + Bases Made Easy! Part 1 - What the Heck is an Acid or Base? - Organic Chemistry
Trick to identify Acid and Base|| class 8,9,10,11,12|| Acid base trick
How to Find the Conjugate Base of an Acid Shortcut, Practice Problems, & Examples
Acids and Bases Grade 11 Past paper exam question PART 1
How to Identity Which Acid is Stronger than Other || Acidic Strength
Checking battery acid levels
Predict Direction of Acid Base Rxn
Identify Acid and Base Grade 11
Acid and Base | Acids, Bases & pH | Video for Kids
How to Determine What Side Acid-Base Equilibria Favor?
How To Memorize The Strong Acids | Trick for Strong Acid & Weak Acid #shorts #reels #jee
Given salt, find acid and base | Chemistry | Khan Academy
Most Common Sign of Low Stomach Acid is...
Acid Bases Indicator Test | Chemistry Demonstration | Grade 7-12
How to identify conjugate acid and base pairs in chemistry
Metals react with acids to produce salt and Hydrogen | Acid & Bases | Chemistry
A Simple Test for Low Stomach Acid
How to determine SALT FORMATION (molecular formula) in acid-base reactions | Chemistry with Cat
Is Your Gold Real? How To Test 10K Gold with Acid - Real Results!
Комментарии
 0:02:34
0:02:34
 0:04:09
0:04:09
 0:11:39
0:11:39
 0:05:49
0:05:49
 0:03:00
0:03:00
 0:04:57
0:04:57
 0:06:50
0:06:50
 0:02:35
0:02:35
 0:21:21
0:21:21
 0:04:51
0:04:51
 0:06:06
0:06:06
 0:09:10
0:09:10
 0:08:59
0:08:59
 0:03:13
0:03:13
 0:08:43
0:08:43
 0:00:49
0:00:49
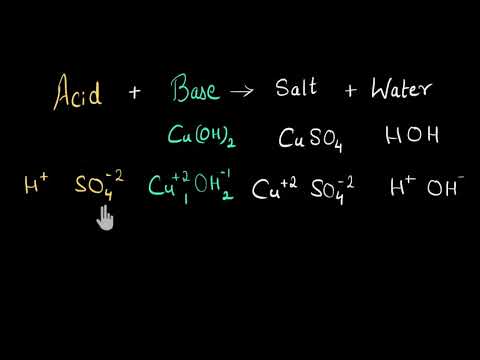 0:08:02
0:08:02
 0:06:04
0:06:04
 0:10:06
0:10:06
 0:06:04
0:06:04
 0:01:50
0:01:50
 0:11:12
0:11:12
 0:04:22
0:04:22
 0:04:00
0:04:00