filmov
tv
Ammonia and Ammonium in the Aquarium
Показать описание
*FREE* downloadable content!
Join The DIY AQUAPRO Team !
TWITTER: @DIYAQUAPROS
Ammonia is a nitrogen based compound that is extremely toxic even at very low concentrations. Most aquarium owners are told to keep ammonia concentrations at 0 (undetectable by test kits) and for good reasons. Concentrations as low as .01 mg/L (ppm) can reduce the fitness of your fish, although ammonia toxicity is largely species dependent. Some fish can tolerate higher levels while others prove to be more sensitive.
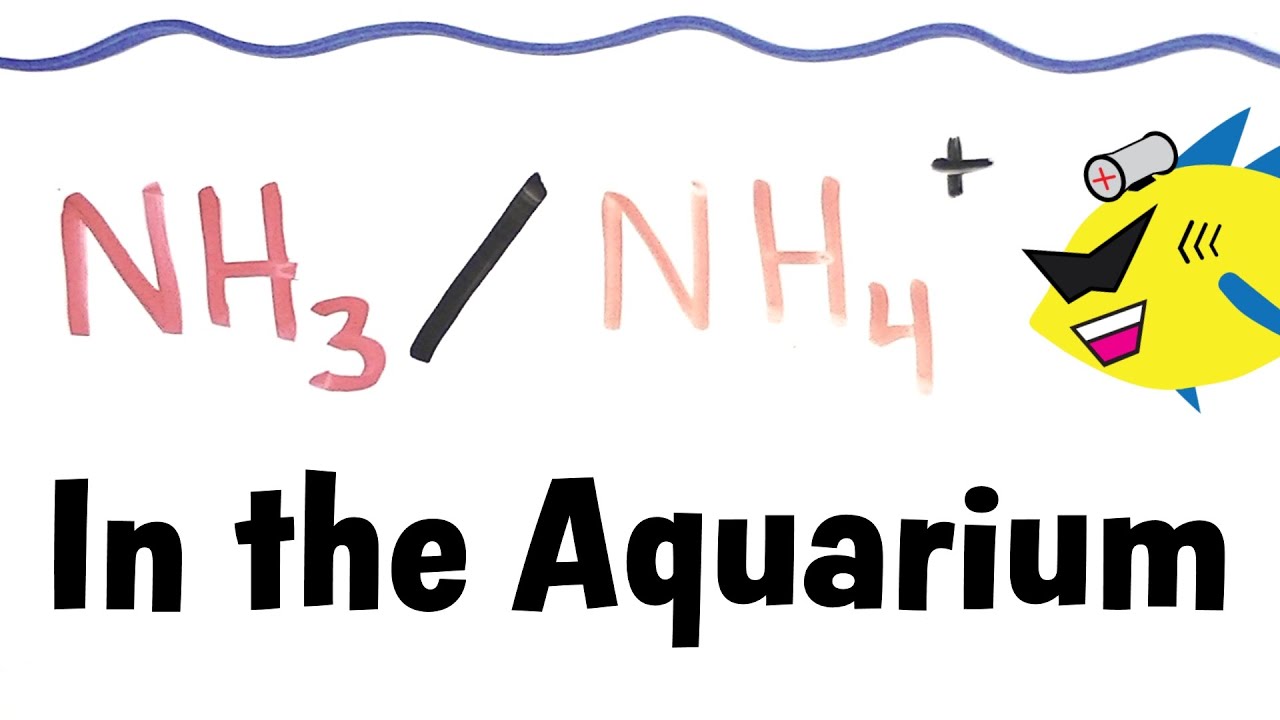
Ammonia is generated by fish waste and the breakdown of organic molecules by bacteria in the aquarium. It exists in an equilibrium with its non-toxic counter part ammonium (NH3/NH4+).The relative amount of each form will be dependent of the pH at any given time.
When the pH is 9.25 there will always be NH3 and NH4+ in equal amounts (1:1). Every time we move 1 full unit of pH there will be a 10 fold increase or decrease of this ratio. If the pH of your aquarium is 8.25 there will be 10x more NH4+ than NH3. If the pH is 7.25 there will be another 10 fold increase resulting in 100x more NH4+ than NH3.
This means that ammonia toxicity increases and deceases with pH. Aquariums with a higher pH will thus have more nitrogen in the toxic NH3 form and vice verse.
When plants, algae and microorganisms remove ammonium from the water column, the equilibrium between NH3/NH4+ must be restored, thus NH3 molecules will protonate and form NH4+. This action will in turn reduce the amount in the NH3 form and act to reduce the toxic NH3 load.
Join The DIY AQUAPRO Team !
TWITTER: @DIYAQUAPROS
Ammonia is a nitrogen based compound that is extremely toxic even at very low concentrations. Most aquarium owners are told to keep ammonia concentrations at 0 (undetectable by test kits) and for good reasons. Concentrations as low as .01 mg/L (ppm) can reduce the fitness of your fish, although ammonia toxicity is largely species dependent. Some fish can tolerate higher levels while others prove to be more sensitive.
Ammonia is generated by fish waste and the breakdown of organic molecules by bacteria in the aquarium. It exists in an equilibrium with its non-toxic counter part ammonium (NH3/NH4+).The relative amount of each form will be dependent of the pH at any given time.
When the pH is 9.25 there will always be NH3 and NH4+ in equal amounts (1:1). Every time we move 1 full unit of pH there will be a 10 fold increase or decrease of this ratio. If the pH of your aquarium is 8.25 there will be 10x more NH4+ than NH3. If the pH is 7.25 there will be another 10 fold increase resulting in 100x more NH4+ than NH3.
This means that ammonia toxicity increases and deceases with pH. Aquariums with a higher pH will thus have more nitrogen in the toxic NH3 form and vice verse.
When plants, algae and microorganisms remove ammonium from the water column, the equilibrium between NH3/NH4+ must be restored, thus NH3 molecules will protonate and form NH4+. This action will in turn reduce the amount in the NH3 form and act to reduce the toxic NH3 load.
Комментарии
 0:03:15
0:03:15
 0:04:13
0:04:13
 0:03:08
0:03:08
 0:04:04
0:04:04
 0:05:23
0:05:23
 0:00:41
0:00:41
 0:00:23
0:00:23
 0:01:24
0:01:24
 0:34:39
0:34:39
 0:01:01
0:01:01
 0:05:42
0:05:42
 0:03:15
0:03:15
 0:02:29
0:02:29
 0:00:36
0:00:36
 0:02:21
0:02:21
 0:01:45
0:01:45
 0:01:21
0:01:21
 0:02:49
0:02:49
 0:01:21
0:01:21
 0:04:22
0:04:22
 0:02:42
0:02:42
 0:00:16
0:00:16
 0:10:00
0:10:00
 0:08:56
0:08:56