filmov
tv
Ammonia vs the Ammonium Ion (NH3 vs NH4 +)

Показать описание
A brief explanation of the differences between Ammonia (NH3) and the Ammonium Ion (NH4+) including Lewis structures, molecular geometry, and bond angles.
In comparing and contrasting Ammonia and the Ammonium ion:
• Ammonia is a strong smelling substance. The Ammonium ion has no odor.
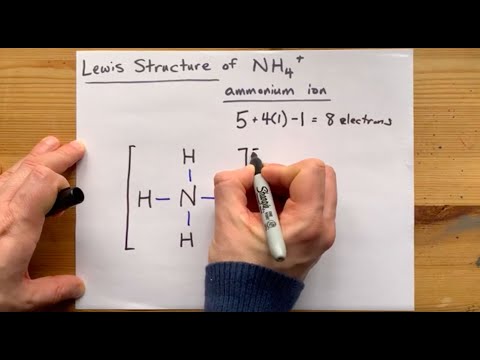
• Both Ammonia and the Ammonium ion have eight valence electrons.
• Ammonia has a trigonal pyramidal molecular geometry. This is because of the lone pair of electrons.
• The Ammonium ion has a tetrahedral molecular geometry since it has four single bonds to the H atoms and no lone pairs.
• Ammonia (NH3) is a single molecule. The Ammonium ion (NH4+) exists bonded to other atoms, like Cl in the compound NH4Cl, or surrounded by water molecules when dissolved in water.
Resources for the Ammonia (NH3):
Resources for the Ammonium Ion (NH4+):
Other Resources:
In comparing and contrasting Ammonia and the Ammonium ion:
• Ammonia is a strong smelling substance. The Ammonium ion has no odor.
• Both Ammonia and the Ammonium ion have eight valence electrons.
• Ammonia has a trigonal pyramidal molecular geometry. This is because of the lone pair of electrons.
• The Ammonium ion has a tetrahedral molecular geometry since it has four single bonds to the H atoms and no lone pairs.
• Ammonia (NH3) is a single molecule. The Ammonium ion (NH4+) exists bonded to other atoms, like Cl in the compound NH4Cl, or surrounded by water molecules when dissolved in water.
Resources for the Ammonia (NH3):
Resources for the Ammonium Ion (NH4+):
Other Resources:
Ammonia vs the Ammonium Ion (NH3 vs NH4 +)
Ammonia vs the Ammonium Ion (NH3 vs NH4 +)
Ammonia vs the Ammonium Ion (NH3 vs NH4 +)
Ammonia vs. Ammonium
Is the ammonium ion NH4+ an acid or a base?
All about Ammonia verse Ammonium
What is Ammonia Nitrogen and how to measure NH4-N in water?
Ammonia and Ammonium in the Aquarium
Salt Analysis of Ammonium Carbonate For Class 11th & 12th Chemistry Qualitative Analysis
Lewis Structure of NH4+, Ammonium ion
The shapes of nitrogen, ammonia and ammonium ion explained via quantum-chemistry calculation
Confusing w/ nomenclature? AMMONIA vs AMMONIUM _Memorization Techniques #3
Ammonium ion test with Practical Guru Monu Sharma
THe difference netween NH4 und NO3
Testing for ammonium ions
Nessler's reagent for Ammonia
Structure and Formation of NH4+ Ammonium Ion | Inorganic Chemistry
ammonium hydroxide is a base #chemistry #chemistryexperiments #chemicalreaction #practical
Dative Covalent Bonding. Donor Atom. Acceptor Atom. Ammonia Borane. Ammonium Ion. Hydronium Ion.
Testing for the ammonium ion
Ammonia + Hydrochloric Acid = 💨🧂💨! #shorts
Ammonium Molybdate Preparation
formula and structure of ammonia and ammonium Ion l chemistry l
What happens when an ammonium salt is heated with Sodium hydroxide solution?
Комментарии
 0:03:15
0:03:15
 0:04:04
0:04:04
 0:04:04
0:04:04
 0:03:42
0:03:42
 0:06:05
0:06:05
 0:02:42
0:02:42
 0:02:34
0:02:34
 0:04:13
0:04:13
 0:10:05
0:10:05
 0:02:29
0:02:29
 0:11:12
0:11:12
 0:00:49
0:00:49
 0:00:58
0:00:58
 0:00:53
0:00:53
 0:01:41
0:01:41
 0:00:16
0:00:16
 0:03:15
0:03:15
 0:00:17
0:00:17
 0:01:16
0:01:16
 0:02:42
0:02:42
 0:00:56
0:00:56
 0:00:16
0:00:16
 0:00:58
0:00:58
 0:00:16
0:00:16