filmov
tv
Reaction of NH3 (g) + HCl (g) Can two gases make a solid? 🌪

Показать описание
If we take NH3 gas (a colorless gas) and mix it with HCl gas (another colorless gas) a reaction producing solid NH4Cl will take place. Where the NH3 and HCl gas come in contact with each other a white cloud forms. This white cloud is made up of very small solid crystals of solid NH4Cl (Ammonium chloride).
The chemical reaction can be written as:
NH3 (g) + HCl (gas) = NH4Cl (s)
As a word equation it would be written:
Ammonia gas plus Hydrogen chloride gas yields solid Ammonium chloride:
The particles of NH4Cl that form a so small that they look like smoke and take a long time to settle. After some time we could collect the Ammonium chloride crystals. If we were to heat the crystals up we could cause the NH4Cl to decompose back into NH3 (g) + HCl (g).
In answer to our question, two gases can react to form a solid. In this case NH3 (g) + HCl (g) produces the NH4Cl (s).
The chemical reaction can be written as:
NH3 (g) + HCl (gas) = NH4Cl (s)
As a word equation it would be written:
Ammonia gas plus Hydrogen chloride gas yields solid Ammonium chloride:
The particles of NH4Cl that form a so small that they look like smoke and take a long time to settle. After some time we could collect the Ammonium chloride crystals. If we were to heat the crystals up we could cause the NH4Cl to decompose back into NH3 (g) + HCl (g).
In answer to our question, two gases can react to form a solid. In this case NH3 (g) + HCl (g) produces the NH4Cl (s).
Reaction of NH3 (g) + HCl (g) Can two gases make a solid? 🌪
The Reaction of HCl(g) with NH3(g) to Produce NH4Cl Smoke
chemical reactions between NH3 gas and HCl gas ......📝📝📝🤔🤔🤔💭💭💭 #chemicalexperiments #jeechemistry...
Reaction of NH3 g + HCl g Can two gases make a solid 🌪
Type of Reaction for NH3 + HCl = NH4Cl
Ammonia and hcl reaction
Reaction between NO2(g) + NH3(g)
NH4HS(s) ⇌ NH3(g) + H2S(g)In the above reaction if the pressure at equilibrium and at 300K is 100atm...
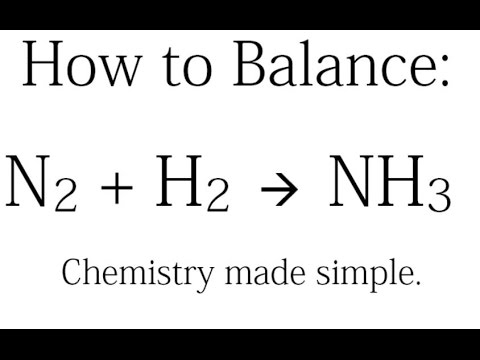
How to Balance: N2 + H2 = NH3 (Synthesis of Ammonia)
How many grams of O2 are needed to react completely with 25.00 g of ammonia, NH3
Sodium(Na)+Ammonia gas(NH3).The Balanced chemical Equation between Sodium & Ammonia gas
Ammonia reacts with oxygen according to the equation 4 NH3 g + 5 O2 g + 6 H2O g Delta H rxn= 906 k
Making NH3 (g) from NH3 (aq)
50.0Kg of N2 (g) and 10.0Kg of H2 (g) are mixed to produce NH3 (g). Calculate the amount of NH3 (g)
How to balance N2(g) + H2(g) → NH3(g)
Ammonia Gas on reaction with HCl produces a dense white fumes.
How to Balance: NH3 + O2 = NO + H2O
Balance NH3 + HCl = NH4Cl (Ammonia and Hydrochloric Acid)
Chemical react in mid air (hydrochloric acid and Ammonia)
NH3 (g) + HCl (g) → NH4Cl (s)
Hows to balance N2H4(l) → NH3(g) + N2(g)
Consider the reaction below: NH3 (g) + HCl (g) → NH4Cl (s), given the following table of thermody…...
Ammonia vs HCl Experiment #shorts #sjw #scienceexperiments #chemistry #experiment #class10
How to Balance NH3 + O2 = NO2 + H2O (Ammonia + Oxygen gas)
Комментарии
 0:01:21
0:01:21
 0:00:37
0:00:37
 0:00:15
0:00:15
 0:01:21
0:01:21
 0:01:11
0:01:11
 0:00:15
0:00:15
 0:01:11
0:01:11
 0:03:43
0:03:43
 0:01:00
0:01:00
 0:02:28
0:02:28
 0:00:55
0:00:55
 0:04:21
0:04:21
 0:01:49
0:01:49
 0:12:23
0:12:23
 0:02:32
0:02:32
 0:00:16
0:00:16
 0:02:41
0:02:41
 0:01:26
0:01:26
 0:00:25
0:00:25
 0:00:15
0:00:15
 0:04:05
0:04:05
 0:00:33
0:00:33
 0:00:48
0:00:48
 0:02:51
0:02:51