filmov
tv
Measuring Enthalpy Changes - Reactivity 1.1 [2025] IB CHEM SL/HL

Показать описание
1.1.1—Chemical reactions involve a transfer of energy between the system and the
surroundings, while total energy is conserved.
1.1.2—Reactions are described as endothermic or exothermic, depending on the
direction of energy transfer between the system and the surroundings.
1.1.3—The relative stability of reactants and products determines whether reactions are
endothermic or exothermic.
1.1.4—The standard enthalpy change for a chemical reaction, ΔH ⦵, refers to the heat transferred at constant pressure under standard conditions and states. It can be determined from the change in temperature of a pure substance.
surroundings, while total energy is conserved.
1.1.2—Reactions are described as endothermic or exothermic, depending on the
direction of energy transfer between the system and the surroundings.
1.1.3—The relative stability of reactants and products determines whether reactions are
endothermic or exothermic.
1.1.4—The standard enthalpy change for a chemical reaction, ΔH ⦵, refers to the heat transferred at constant pressure under standard conditions and states. It can be determined from the change in temperature of a pure substance.
Measuring Enthalpy Changes - Reactivity 1.1 [2025] IB CHEM SL/HL
IB Chemistry SL | Reactivity 1.1 - Measuring Enthalpy Changes | 19M.2.SL.TZ1.8c
Enthalpy Changes [IB Chemistry SL/HL]
IB Chemistry SL | Reactivity 1.1 - Measuring Enthalpy Changes | 22M.1A.SL.TZ1.13
IB Chemistry SL | Reactivity 1.1 - Measuring Enthalpy Changes | 20N.1A.SL.TZ0.28
IB Chemistry SL | Reactivity 1.1 - Measuring Enthalpy Changes | 19N.1A.SL.TZ0.13
R1.1.4 Enthalpy and enthalpy change
IB Chemistry SL | Reactivity 1.1 - Measuring Enthalpy Changes | 19M.1A.SL.TZ1.15
GCSE Chemistry - Exothermic and Endothermic Reactions #43
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Problems Chemistry
Enthalpy of reaction | Thermodynamics | AP Chemistry | Khan Academy
Energy & Chemical Change| L4: Calculating Enthalpy Change 'Part 1' @EasyChemistry4all
The EASIEST Method For Solving Hess Cycles
34.7 Determine enthalpy change of reaction
Energy balance in English | 44 | Introduction to calculating enthalpy change for reactive systems
Measuring Enthalpy Changes (IB Chemistry R1.1)
Enthalpy Change Calculation Based on a practical [AS]
Standard Enthalpy Changes - combustion and formation (A-Level Chemistry)
Measuring enthalpy change part 4 (enthalpy graphs)
Standard enthalpy changes
Energy balance in Arabic | 44 | Introduction to calculating enthalpy change for reactive systems
5.1 Calculate enthalpy change for a reaction using experimental data (mcdeltaT) [SL IB Chemistry]
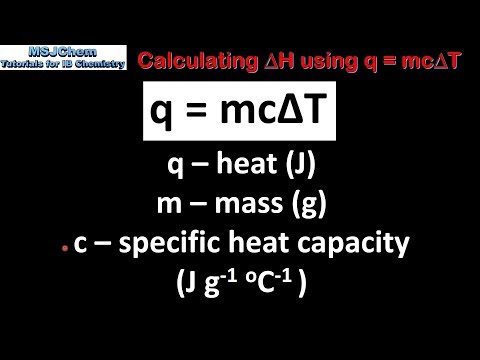
R1.1.4 Calculating ΔH using q = mcΔT
Enthalpies of solution
Комментарии
 0:09:31
0:09:31
 0:05:11
0:05:11
 0:11:56
0:11:56
 0:04:59
0:04:59
 0:04:28
0:04:28
 0:05:01
0:05:01
 0:01:29
0:01:29
 0:02:50
0:02:50
 0:05:21
0:05:21
 0:16:42
0:16:42
 0:04:17
0:04:17
 0:27:55
0:27:55
 0:13:46
0:13:46
 0:22:52
0:22:52
 0:16:35
0:16:35
 0:18:35
0:18:35
 0:04:48
0:04:48
 0:10:17
0:10:17
 0:10:30
0:10:30
 0:10:14
0:10:14
 0:17:34
0:17:34
 0:09:30
0:09:30
 0:03:35
0:03:35
 0:07:38
0:07:38