filmov
tv
Calculate Amount of Reactant Needed (Example)

Показать описание
Calculate Amount of Reactant Needed (Example)
Calculating masses in reactions - p27 (Chem)
Determining amount of other reactant needed given the amount of one reactant
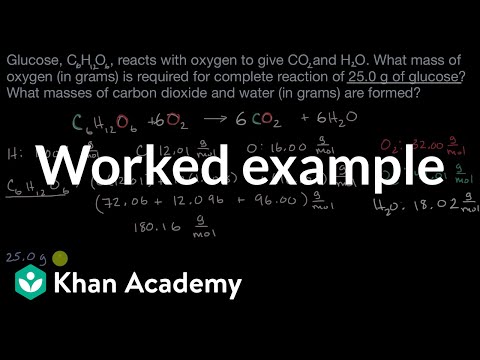
Worked example: Calculating amounts of reactants and products | AP Chemistry | Khan Academy
Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems
Stoichiometry Mole to Mole Conversions - Molar Ratio Practice Problems
Find the Amount of Excess Reactant (+ Example)
Reacting Masses - Worked Example
Mass balance in English | 34 | Intro to MB for reactive systems - Reaction stoichiometry
Stoichiometric calculations. (Calculating the mass of reactant used and the volume of gas produced)
Stoichiometry | Mole to mole | Grams to grams | Mole to grams | Grams to mole | Mole ratio
How to Find the Mole Ratio to Solve Stoichiometry Problems
Reverse % yield: How to work out the mass of reactant needed to produce a given mass of product
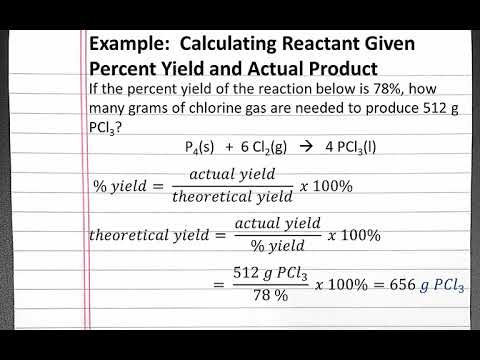
CHEMISTRY 101 - Calculating Reactant Given Percent Yield and Actual Product
Introduction to Limiting Reactant and Excess Reactant
Limiting Reactant Practice Problems
Convert Molar Mass to Moles (2021)
How To Find The Amount of Excess Reactant That Is Left Over - Chemistry
Worked example: Calculating the amount of product formed from a limiting reactant | Khan Academy
GCSE Chemistry - How to Find the Volume of a Gas #28
Stoichiometry - calculating volume of reactant
Solution Stoichiometry - Finding Molarity, Mass & Volume
GCSE Chemistry - What is a Limiting Reactant? Limiting/Excess Reactants Explained #27
Stoichiometry Example: Calculating the amount of excess reactant
Комментарии
 0:03:42
0:03:42
 0:05:54
0:05:54
 0:03:06
0:03:06
 0:12:06
0:12:06
 0:25:16
0:25:16
 0:12:11
0:12:11
 0:05:37
0:05:37
 0:06:48
0:06:48
 0:13:43
0:13:43
 0:10:44
0:10:44
 0:17:16
0:17:16
 0:08:44
0:08:44
 0:10:59
0:10:59
 0:01:53
0:01:53
 0:16:58
0:16:58
 0:18:52
0:18:52
 0:01:25
0:01:25
 0:13:43
0:13:43
 0:06:22
0:06:22
 0:06:58
0:06:58
 0:08:49
0:08:49
 0:23:11
0:23:11
 0:04:16
0:04:16
 0:02:47
0:02:47