filmov
tv
Worked example: Calculating amounts of reactants and products | AP Chemistry | Khan Academy

Показать описание
Keep going! Check out the next lesson and practice what you’re learning:
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
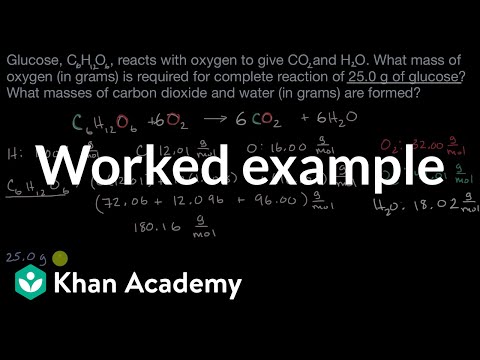
Worked example: Calculating amounts of reactants and products | AP Chemistry | Khan Academy
Worked example: Calculating the amount of product formed from a limiting reactant | Khan Academy
Calculating masses in reactions - p27 (Chem)
Worked example: Calculating molar mass and number of moles | AP Chemistry | Khan Academy
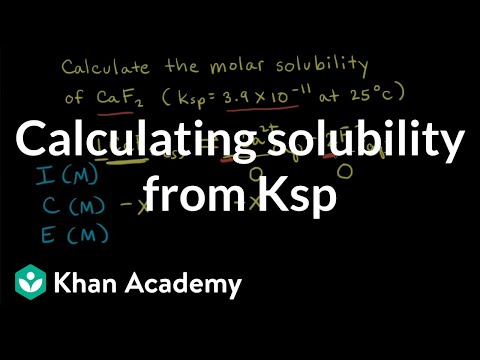
Worked example: Calculating solubility from Kₛₚ | Equilibrium | AP Chemistry | Khan Academy
Calculating Mole Grade 10 | Part 1
S1.4.3 Calculating amount (in mol) of substance
Worked example: Calculating mass percent | AP Chemistry | Khan Academy
How to use an Excel Data Table | Calculate Maturity Amount at Multiple % and Years #trending #shorts
Calculating Working Capital in Excel
Calculating correct injections volumes
Worked examples: Calculating equilibrium constants | Equilibrium | AP Chemistry | Khan Academy
Calculating Safety Stock: Protecting Against Stock Outs
Worked example: Calculating concentration using the Beer–Lambert law | AP Chemistry | Khan Academy
Worked example of calculating whether a reaction goes forward or backward
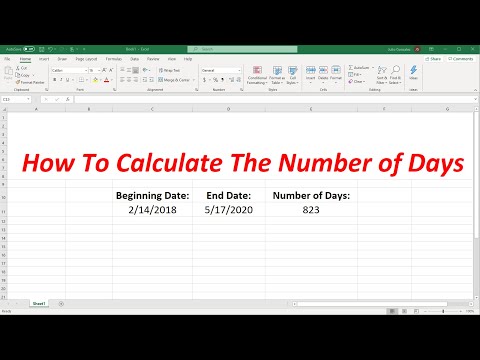
How To Calculate The Number of Days Between Two Dates In Excel
Worked example: calculating ion charge | High school chemistry | Khan Academy
How To Calculate Square Metres - DIY At Bunnings
Worked example: Calculating the mass of a substance in a mixture | AP Chemistry | Khan Academy
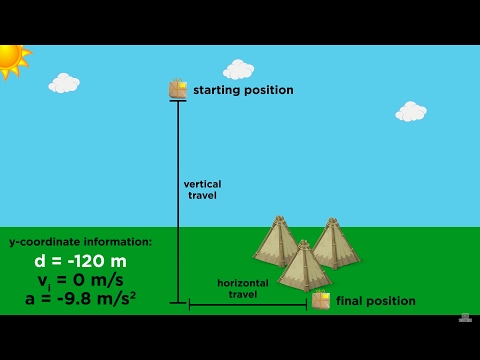
Kinematics Part 4: Practice Problems and Strategy
Finding a Percent of a Number | Calculating Percentages
Worked example: Calculating partial pressures | AP Chemistry | Khan Academy
Calculating CFM or Velocity from Area
ENE 483: Lime Softening Example: Calculating Stoichiometric Amounts of Lime and Soda ash
Комментарии
 0:12:06
0:12:06
 0:06:22
0:06:22
 0:05:54
0:05:54
 0:05:49
0:05:49
 0:04:52
0:04:52
 0:11:46
0:11:46
 0:05:54
0:05:54
 0:05:38
0:05:38
 0:00:52
0:00:52
 0:00:18
0:00:18
 0:03:19
0:03:19
 0:08:12
0:08:12
 0:06:17
0:06:17
 0:03:48
0:03:48
 0:04:29
0:04:29
 0:01:18
0:01:18
 0:02:47
0:02:47
 0:02:03
0:02:03
 0:05:21
0:05:21
 0:06:46
0:06:46
 0:06:27
0:06:27
 0:08:03
0:08:03
 0:05:25
0:05:25
 0:21:30
0:21:30