filmov
tv
Convert Molar Mass to Moles (2021)
Показать описание
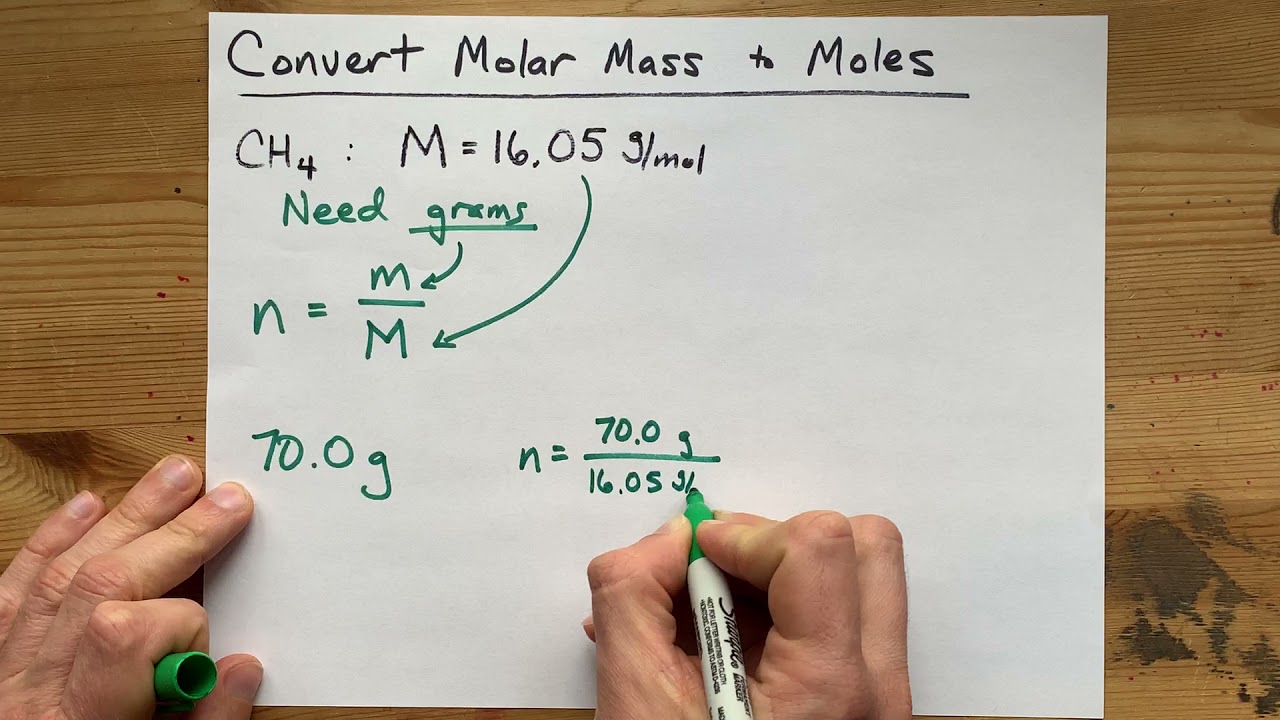
To convert a molar mass to moles, you HAVE to know the amount of substance, given in Grams. This is because molar mass is how many grams each MOLE of the substance weighs, like a ratio, so you need at least one other piece of information to solve for moles.
mass = moles TIMES molar mass.
mass = moles TIMES molar mass.
Convert Molar Mass to Moles (2021)
Converting Between Grams and Moles
Converting Grams to Moles Using Molar Mass | How to Pass Chemistry
Mole Conversions Made Easy: How to Convert Between Grams and Moles
How To Convert Grams To Moles - VERY EASY!
Convert Mass To Moles - Practice - 1
Worked example: Calculating molar mass and number of moles | AP Chemistry | Khan Academy
Molar Conversions: Grams to Moles and Moles to Grams
. cal the Mole fraction of sodium hydroxide in 10%w/w NaOH Solution jee, neet25 ,cbse12,ipe 2nd yr
How To Convert Between Moles, Atoms, and Grams In Chemistry - QUICK & SIMPLE!
Introduction to Moles
How to Convert Grams to Moles (with Molar Mass)
Converting Between Moles, Atoms, and Molecules
Conversion of moles to gram & grams to moles
Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems
Converting Between Grams and Moles (Part 2)
How To Calculate The Molar Mass of a Compound - Quick & Easy!
The Mole 5 - Converting Moles of Compound to Mass
The Mole: Avogadro's Number and Stoichiometry
Chem 3.9b: Molar conversion - mass to moles
How To Convert Mass to Moles Using Molar Mass
Converting from Atoms to Moles
Moles to Grams in One minute
Interconverting Masses, Moles and Numbers of Particles - Chemistry Tutorial
Комментарии
 0:01:25
0:01:25
 0:10:47
0:10:47
 0:04:56
0:04:56
 0:07:25
0:07:25
 0:13:17
0:13:17
 0:02:29
0:02:29
 0:05:49
0:05:49
 0:05:35
0:05:35
 0:03:22
0:03:22
 0:19:08
0:19:08
 0:05:16
0:05:16
 0:05:14
0:05:14
 0:14:00
0:14:00
 0:01:37
0:01:37
 0:25:16
0:25:16
 0:06:13
0:06:13
 0:11:20
0:11:20
 0:06:39
0:06:39
 0:06:06
0:06:06
 0:10:13
0:10:13
 0:00:54
0:00:54
 0:02:25
0:02:25
 0:00:59
0:00:59
 0:06:17
0:06:17