filmov
tv
Structure of Atom - Chemistry Notes Info

Показать описание
11 Class Chapter 2- Structure of Atom
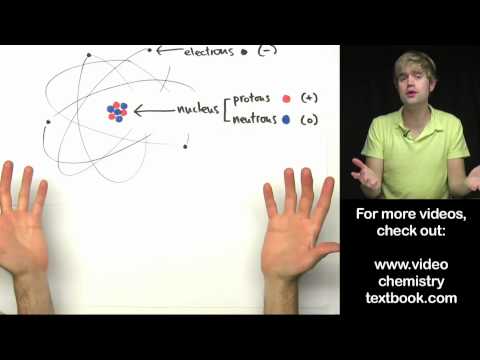
Atomic Theory of matter
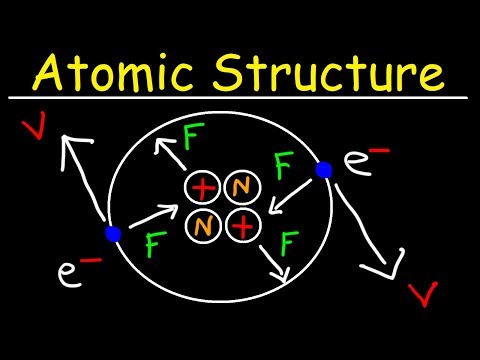
According to this theory , atom is the ultimate particle of matter , also known as Dalton’s Atomic theory 1808.
Cathode ray discharge tube experiments: -
1. Cathode rays start from cathode and move toward anode.
2. These rays are not visible but there behavior can be observed with fluorescent or phosphorus sent material.
3. In the absence of magnetic or electric field these travels in strait lines
4. In the presence or magnetic or electric field the behavior of cathode rays in similar TO Negatively charged particles which suggest that these rays contain negatively charge particles called electron
5. Cathode rays do not depend on the martial of the electrode and nature of the gas in the tube so electron are basic constituent of all atoms.
Charge to mass ratio of electron
Measured by J. J. Thomson 1897.
By using cathode ray tube ; applying electrical & magnetic field perpendicular to each other also perpendicular to path of electrons.
He proposed deviation of particles from their path in presence of magnetic or electrical field depend upon the following
1. Magnetic of – ve charge on particle
i.e. it magnitude of charge on particles is greater than interaction with magnetic or electric field is greater so deflection is also grater.
2. Mass of particles
i.e. particle is lighter then deflection is greater.
3. Strength of magnetic or electric field.
0:00 Introduction
0:23 Cathode Ray Discharge Tube Experiments
3:04 Charge of Electron
3:26 Mass of Electron
3:45 Discovery of Proton
5:10 Thomson Model of Atom
5:57 Rutherford's Nuclear Model of Atom
6:26 Rutherford Observed That
6:54 From above observations he concludes the structure of atom.
7:45 On the basis of observation & conclusion Rutherford proposed model of atom as
11:02 Photo Electric Effect
14:23 Dual Behaviour of Matter
15:00 Heisenberg's Uncertainty Principle
15:46 Quantum Mechanical Model of Atom
16:28 Principle Quantum Number 'N'
17:06 Azimuthal Quantum No. 'P'
18:32 Electron Spin Quantum (Mc)
18:58 Aufbau Principle
19:55 Hund's Rule of Maximum Multiplicity According to this rule pairing of
Atomic Theory of matter
According to this theory , atom is the ultimate particle of matter , also known as Dalton’s Atomic theory 1808.
Cathode ray discharge tube experiments: -
1. Cathode rays start from cathode and move toward anode.
2. These rays are not visible but there behavior can be observed with fluorescent or phosphorus sent material.
3. In the absence of magnetic or electric field these travels in strait lines
4. In the presence or magnetic or electric field the behavior of cathode rays in similar TO Negatively charged particles which suggest that these rays contain negatively charge particles called electron
5. Cathode rays do not depend on the martial of the electrode and nature of the gas in the tube so electron are basic constituent of all atoms.
Charge to mass ratio of electron
Measured by J. J. Thomson 1897.
By using cathode ray tube ; applying electrical & magnetic field perpendicular to each other also perpendicular to path of electrons.
He proposed deviation of particles from their path in presence of magnetic or electrical field depend upon the following
1. Magnetic of – ve charge on particle
i.e. it magnitude of charge on particles is greater than interaction with magnetic or electric field is greater so deflection is also grater.
2. Mass of particles
i.e. particle is lighter then deflection is greater.
3. Strength of magnetic or electric field.
0:00 Introduction
0:23 Cathode Ray Discharge Tube Experiments
3:04 Charge of Electron
3:26 Mass of Electron
3:45 Discovery of Proton
5:10 Thomson Model of Atom
5:57 Rutherford's Nuclear Model of Atom
6:26 Rutherford Observed That
6:54 From above observations he concludes the structure of atom.
7:45 On the basis of observation & conclusion Rutherford proposed model of atom as
11:02 Photo Electric Effect
14:23 Dual Behaviour of Matter
15:00 Heisenberg's Uncertainty Principle
15:46 Quantum Mechanical Model of Atom
16:28 Principle Quantum Number 'N'
17:06 Azimuthal Quantum No. 'P'
18:32 Electron Spin Quantum (Mc)
18:58 Aufbau Principle
19:55 Hund's Rule of Maximum Multiplicity According to this rule pairing of
Комментарии
 0:11:45
0:11:45
 0:05:22
0:05:22
 0:30:31
0:30:31
 0:02:20
0:02:20
 0:07:44
0:07:44
 0:04:32
0:04:32
 0:07:20
0:07:20
 0:18:49
0:18:49
 0:02:01
0:02:01
 0:13:18
0:13:18
 0:05:23
0:05:23
 0:09:42
0:09:42
 0:08:42
0:08:42
 0:09:49
0:09:49
 0:10:37
0:10:37
 1:57:06
1:57:06
 1:04:43
1:04:43
 0:11:19
0:11:19
 0:00:12
0:00:12
 0:07:17
0:07:17
 0:10:19
0:10:19
 5:13:38
5:13:38
 3:11:55
3:11:55
 0:35:31
0:35:31