filmov
tv
Ionic Bonding Between Metals and Non-Metals (Atomic Bonding Mechanisms)

Показать описание
A tutorial to explain how ionic bonds form between metals and non-metals.
In order to become stable, metal atoms lose electrons. Non-metals become stable by gaining electrons. The metal donates electrons to the non-metal so that both form ions (a positive metal ion and a negative non-metal ion). As a result, the positively and negatively charged ions are attracted to each other, forming the ionic bond.
 0:04:12
0:04:12
 0:09:15
0:09:15
 0:03:03
0:03:03
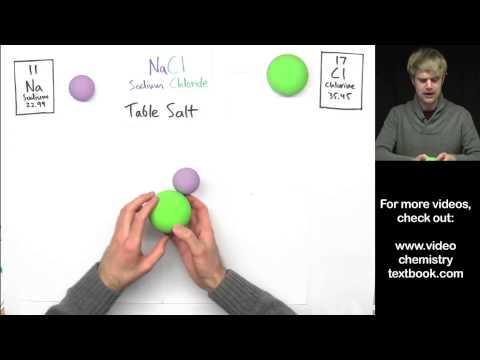 0:07:20
0:07:20
 0:11:50
0:11:50
 0:03:34
0:03:34
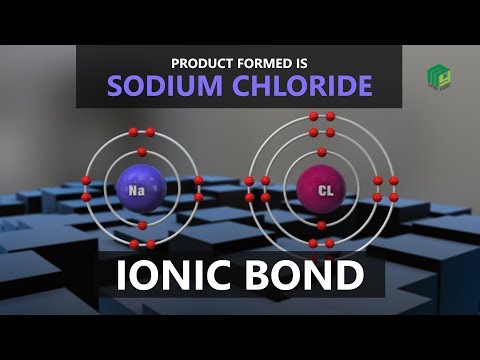 0:01:47
0:01:47
 0:00:15
0:00:15
 0:11:25
0:11:25
 0:03:31
0:03:31
 0:04:40
0:04:40
 0:06:08
0:06:08
 0:09:49
0:09:49
 0:04:30
0:04:30
 0:06:12
0:06:12
 0:01:03
0:01:03
 0:21:57
0:21:57
 0:05:58
0:05:58
 0:06:08
0:06:08
 0:05:12
0:05:12
 0:01:00
0:01:00
 0:07:02
0:07:02
 0:03:21
0:03:21
 0:04:39
0:04:39