filmov
tv
| Ionic Bond | My Inter Academy |
Показать описание
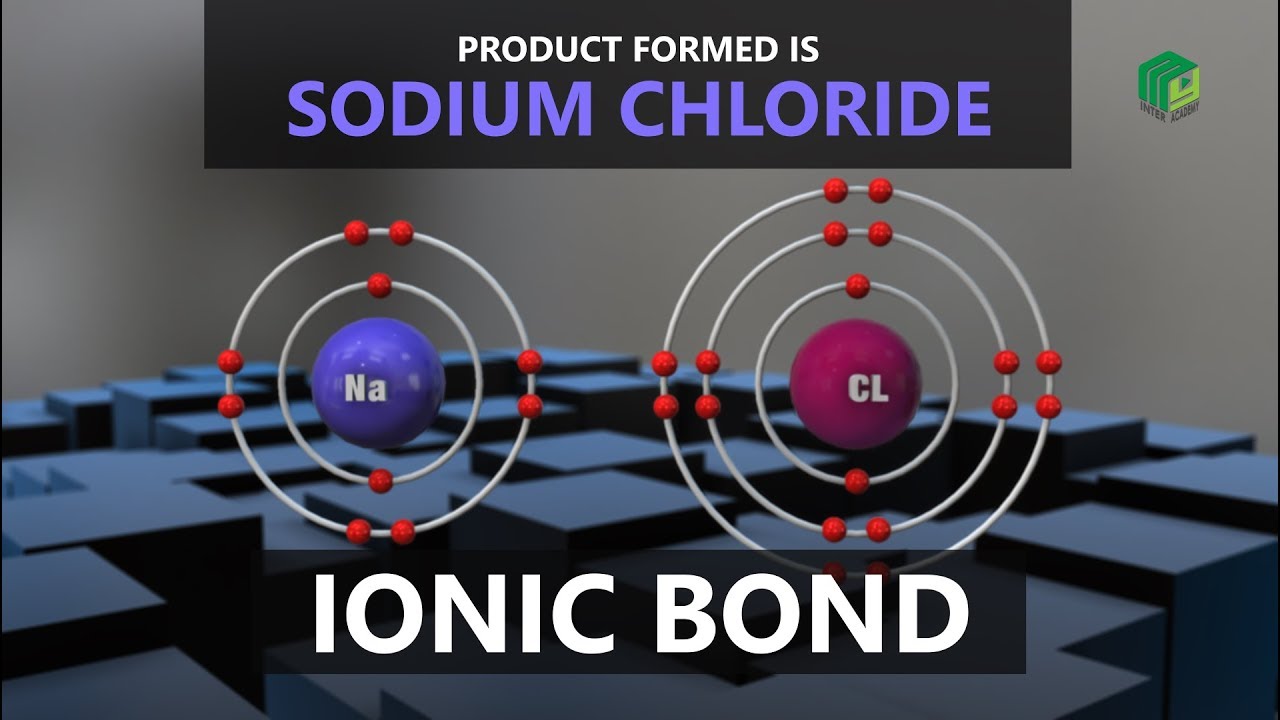
This video explains the formation of Ionic Bond between Sodium and Chlorine.
Ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.
Avail our FREE TRIAL now and satisfy yourself by enjoying our LMS experience!
Here are the links of our official Instagram page and Facebook page:
Ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.
Avail our FREE TRIAL now and satisfy yourself by enjoying our LMS experience!
Here are the links of our official Instagram page and Facebook page:
| Ionic Bond | My Inter Academy |
Ionic Bonding Introduction
Understand Ionic Bond in Animated way
How Ionic Bond is Formed?
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar
Ionic Bonding - p84
8.1 Ionic Bonding | General Chemistry
Ionic Bonding Practice-get started
GRADE 12 ORGANIC MOLECULE (part 2)
Formation of ionic bond in MgF2
Ionic Bonding Part 2
What is Ionic bond #chemical #viral #videos #chemicalreaction#bonding #chrome#amazon
Chemical Bonding - CB 01
How to draw IONIC BONDING of NaCl and MgO
3.07 Ionic Bonding | Cambridge IGCSE Chemistry | GCSE O Level | My Second Teacher
Ionic Bonding: A Love Story l A cartoon guide to formation of ionic bond l Science l Brar Scribbles
Bond Dissociation Energy concept🔥🔥 #shorts #reels #chemistry #jee #neet #tips
Ionic Bonding: A Love Story ❤️ | Cartoon guide to explain the formation of ionic bond | Chemistry...
Covalent Bond explained #shorts #chemistry #jeemains #neet
Ionic Bonding Part 3
Ionic Bonding! (Definition and Examples)
Compare melting point of ionic compounds. View the full video at my channel @ChemistryGuru
difference Between ionic compounds and covalent compounds
Ionic Bonding| Ionic Compounds | Ionic Formulae | Head Start to A level Chemistry
Комментарии
 0:01:47
0:01:47
 0:07:20
0:07:20
 0:04:48
0:04:48
 0:00:54
0:00:54
 0:03:33
0:03:33
 0:06:12
0:06:12
 0:15:52
0:15:52
 0:09:50
0:09:50
 0:16:30
0:16:30
 0:10:51
0:10:51
 0:10:18
0:10:18
 0:00:12
0:00:12
 0:22:15
0:22:15
 0:07:09
0:07:09
 0:05:08
0:05:08
 0:00:46
0:00:46
 0:00:50
0:00:50
 0:00:15
0:00:15
 0:00:26
0:00:26
 0:07:57
0:07:57
 0:07:56
0:07:56
 0:01:00
0:01:00
 0:00:16
0:00:16
 0:22:21
0:22:21