filmov
tv
Law Of Multiple Proportions

Показать описание
Law of multiple proportions
formulated by John Dalton in 1808
statement:
Two elements can combine to form more than one compound, the mass of one element combining with the fixed mass of other are in small whole ratios.
formulated by John Dalton in 1808
statement:
Two elements can combine to form more than one compound, the mass of one element combining with the fixed mass of other are in small whole ratios.
Law of Multiple Proportions Practice Problems, Chemistry Examples, Fundamental Chemical Laws
Law of Multiple Proportions
Law of Multiple Proportions | JEE Chemistry | Anupam Gupta IIT Delhi | Embibe
Law of Multiple Proportions
Law of Multiple Proportions
Chemistry 5.8b Law of Multiple Proportions
Law of Definite Proportions Chemistry Practice Problems - Chemical Fundamental Laws
Law of Multiple Proportions
Who Discovered the Law of Multiple Proportions in Chemistry? : Chemistry & Physics
The Creation of Chemistry - The Fundamental Laws: Crash Course Chemistry #3
Law of Multiple Proportions Explained | CBSE Chemistry | Anupam Gupta IIT Delhi | Embibe
Law of Multiple Proportions: A Ratio Example
ALEKS: Using the Law of Multiple Proportions
Laws of Chemical Combinations - Class 9 Tutorial
Laws of Chemical Combinations
Law of Multiple Proportions 001
Law Of Multiple Proportions
Law of Multiple Proportions - Basic Concepts of Chemistry - Chemistry Class 11
Law of Multiple Proportion #physicswallah
CHEMISTRY 101: The three laws that led to Daltons Atomic Theory
Law of Multiple Proportion | Class 11 Chemistry Chapter 1 | CBSE/JEE/NEET (2022-23)
Multiple proportion law #science #chemistry #cbse #class11chemistry .
what is law of multiple proportion ? #shorts #share #chemistry #class11 #subscribe #like
Unraveling the Law of Multiple Proportions #chemicalreaction #fact #chem #scientificnotation
Комментарии
 0:11:42
0:11:42
 0:04:39
0:04:39
 0:00:59
0:00:59
 0:02:20
0:02:20
 0:10:12
0:10:12
 0:04:01
0:04:01
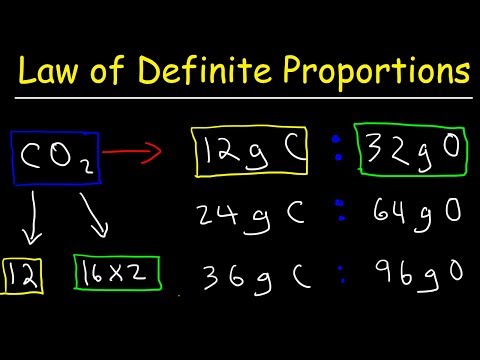 0:10:31
0:10:31
 0:02:21
0:02:21
 0:01:45
0:01:45
 0:10:59
0:10:59
 0:06:06
0:06:06
 0:00:58
0:00:58
 0:05:57
0:05:57
 0:05:14
0:05:14
 0:15:48
0:15:48
 0:05:00
0:05:00
 0:07:36
0:07:36
 0:07:44
0:07:44
 0:00:56
0:00:56
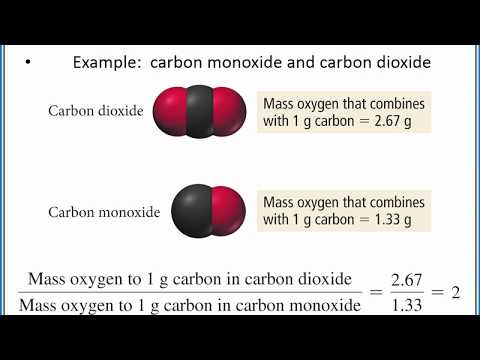 0:04:06
0:04:06
 0:20:59
0:20:59
 0:00:36
0:00:36
 0:01:00
0:01:00
 0:00:53
0:00:53