filmov
tv
Law of Multiple Proportions: A Ratio Example

Показать описание
Imagine you're crafting the ultimate playlist for your next epic road trip. You're mixing and matching songs, trying to find that perfect balance between upbeat tracks and chill vibes. In a way, you're playing DJ with elements from the periodic table when you dive into the Law of Multiple Proportions. This rule is like the secret recipe behind the scenes of chemistry, dictating how elements combine to form different compounds.
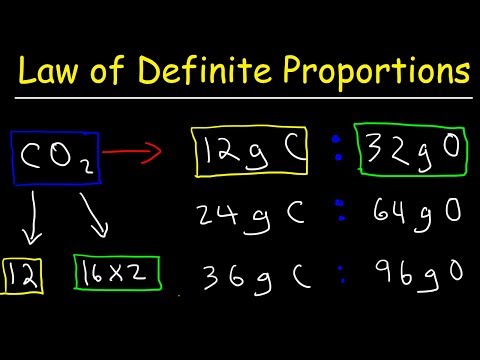
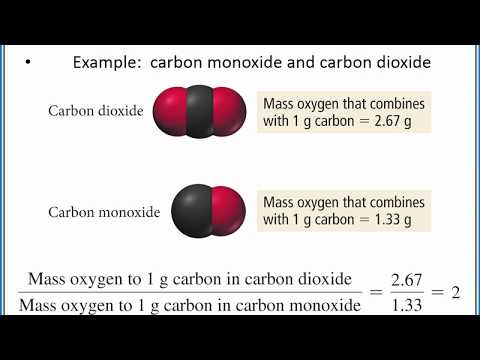
Here’s the scoop: The Law of Multiple Proportions kicks in when two elements come together to party and can form more than one compound. Picture carbon and oxygen. On their own, they’re just chillin'. But when they decide to team up, they can either form carbon monoxide (CO) or carbon dioxide (CO2). This law states that if you're keeping one element constant, the ratios of the masses of the other element in these compounds will relate to each other in a simple whole number ratio.
So, if you take the mass of oxygen that combines with a fixed mass of carbon to form carbon monoxide and compare it with the mass of oxygen that combines with the same mass of carbon to form carbon dioxide, the ratio will always be a simple number (like 1:2 in this case). It’s like saying for every one epic electric guitar solo in your playlist, you might have two killer drum solos, maintaining a balance that keeps the whole journey interesting.
The Law of Multiple Proportions is one of those fundamental principles that shows us chemistry isn’t just random chaos. There’s a specific order and predictability to how things combine, almost like nature’s own playlist follows a beat, ensuring that the world of compounds remains harmoniously in tune.
Here’s the scoop: The Law of Multiple Proportions kicks in when two elements come together to party and can form more than one compound. Picture carbon and oxygen. On their own, they’re just chillin'. But when they decide to team up, they can either form carbon monoxide (CO) or carbon dioxide (CO2). This law states that if you're keeping one element constant, the ratios of the masses of the other element in these compounds will relate to each other in a simple whole number ratio.
So, if you take the mass of oxygen that combines with a fixed mass of carbon to form carbon monoxide and compare it with the mass of oxygen that combines with the same mass of carbon to form carbon dioxide, the ratio will always be a simple number (like 1:2 in this case). It’s like saying for every one epic electric guitar solo in your playlist, you might have two killer drum solos, maintaining a balance that keeps the whole journey interesting.
The Law of Multiple Proportions is one of those fundamental principles that shows us chemistry isn’t just random chaos. There’s a specific order and predictability to how things combine, almost like nature’s own playlist follows a beat, ensuring that the world of compounds remains harmoniously in tune.
Комментарии
 0:11:42
0:11:42
 0:04:39
0:04:39
 0:02:20
0:02:20
 0:10:12
0:10:12
 0:04:01
0:04:01
 0:00:59
0:00:59
 0:01:45
0:01:45
 0:05:57
0:05:57
 0:10:31
0:10:31
 0:02:21
0:02:21
 0:00:58
0:00:58
 0:10:59
0:10:59
 0:05:14
0:05:14
 0:15:48
0:15:48
 0:06:06
0:06:06
 0:04:06
0:04:06
 0:05:00
0:05:00
 0:00:53
0:00:53
 0:01:00
0:01:00
 0:07:36
0:07:36
 0:20:59
0:20:59
 0:00:30
0:00:30
 0:06:50
0:06:50
 0:00:56
0:00:56