filmov
tv
Law of Multiple Proportions

Показать описание
Lesson on the Law of Multiple Proportions, one of the laws that led to modern atomic theory.
Law of Multiple Proportions Practice Problems, Chemistry Examples, Fundamental Chemical Laws
Law of Multiple Proportions
Law of Multiple Proportions Explained | CBSE Chemistry | Anupam Gupta IIT Delhi | Embibe
Chemistry 5.8b Law of Multiple Proportions
Law of Multiple Proportions
Law of Multiple Proportions
The Creation of Chemistry - The Fundamental Laws: Crash Course Chemistry #3
Who Discovered the Law of Multiple Proportions in Chemistry? : Chemistry & Physics
Law of Definite Proportions Chemistry Practice Problems - Chemical Fundamental Laws
ALEKS: Using the Law of Multiple Proportions
Law of Multiple Proportions 001
Laws of Chemical Combinations - Class 9 Tutorial
Law of Multiple Proportions | JEE Chemistry | Anupam Gupta IIT Delhi | Embibe
Law Of Multiple Proportions
Laws of Chemical Combinations
Law of Multiple Proportions - Basic Concepts of Chemistry - Chemistry Class 11
Law of Multiple Proportions
Law of Multiple Proportions | Chemistry | Class 11 | IIT JEE Main + Advanced | NEET | askIITians
Law of Conservation of Mass - Fundamental Chemical Laws, Chemistry
Law of multiple proportions, Class 11, NCERT, Chapter 1, simple expalanation in malayalam.
Law of multiple proportion | Some basic concepts of chemistry | Class 11 Chemistry (CBSE/NCERT)
Law of definite proportions | Atoms and Molecules | Chemistry | Khan Academy
CHEMISTRY 101: The three laws that led to Daltons Atomic Theory
Law of multiple proportion...👍
Комментарии
 0:11:42
0:11:42
 0:04:39
0:04:39
 0:06:06
0:06:06
 0:04:01
0:04:01
 0:02:20
0:02:20
 0:10:12
0:10:12
 0:10:59
0:10:59
 0:01:45
0:01:45
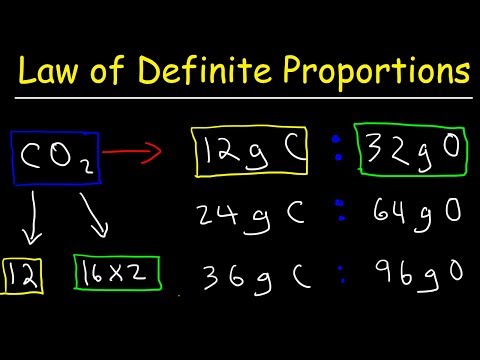 0:10:31
0:10:31
 0:05:57
0:05:57
 0:05:00
0:05:00
 0:05:14
0:05:14
 0:00:59
0:00:59
 0:07:36
0:07:36
 0:15:48
0:15:48
 0:07:44
0:07:44
 0:02:21
0:02:21
 0:08:42
0:08:42
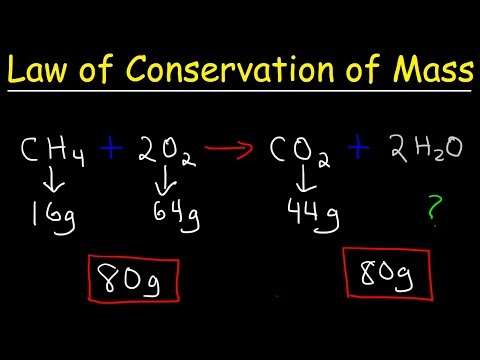 0:03:14
0:03:14
 0:04:15
0:04:15
 0:09:37
0:09:37
 0:02:19
0:02:19
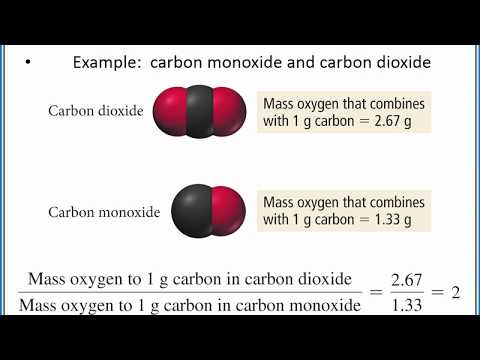 0:04:06
0:04:06
 0:06:49
0:06:49