filmov
tv
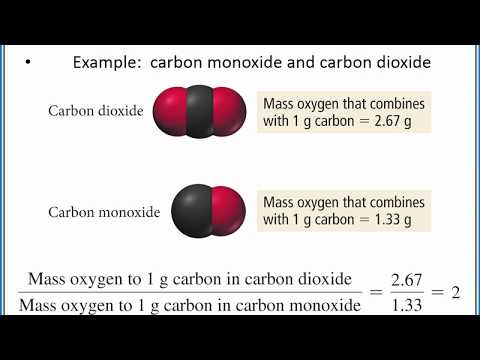
Law of Multiple Proportions

Показать описание
Brief explanation of and quick math problem about the law of multiple proportions.
Law of Multiple Proportions Practice Problems, Chemistry Examples, Fundamental Chemical Laws
Law of Multiple Proportions
Law of Multiple Proportions
Law of Multiple Proportions | JEE Chemistry | Anupam Gupta IIT Delhi | Embibe
Law of Multiple Proportions
Chemistry 5.8b Law of Multiple Proportions
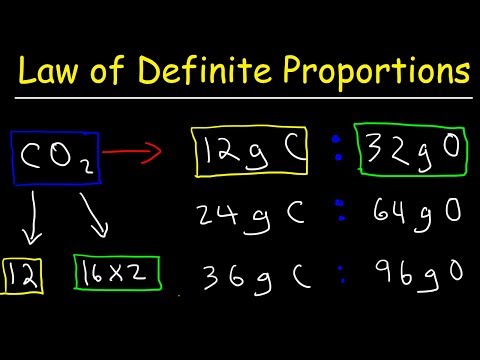
Law of Definite Proportions Chemistry Practice Problems - Chemical Fundamental Laws
Who Discovered the Law of Multiple Proportions in Chemistry? : Chemistry & Physics
Law of Multiple Proportions
Law of Multiple Proportions Explained | CBSE Chemistry | Anupam Gupta IIT Delhi | Embibe
ALEKS: Using the Law of Multiple Proportions
The Creation of Chemistry - The Fundamental Laws: Crash Course Chemistry #3
Law of Multiple Proportions: A Ratio Example
Laws of Chemical Combinations - Class 9 Tutorial
Law of Multiple Proportions 001
Law Of Multiple Proportions
Laws of Chemical Combinations
Law of Multiple Proportions - Basic Concepts of Chemistry - Chemistry Class 11
Law of Multiple Proportion #physicswallah
Law of Multiple Proportion | Class 11 Chemistry Chapter 1 | CBSE/JEE/NEET (2022-23)
CHEMISTRY 101: The three laws that led to Daltons Atomic Theory
Unraveling the Law of Multiple Proportions #chemicalreaction #fact #chem #scientificnotation
law of multiple proportions #tutor360
Law of multiple proportion | Some basic concepts of chemistry | Class 11 Chemistry (CBSE/NCERT)
Комментарии
 0:11:42
0:11:42
 0:04:39
0:04:39
 0:02:20
0:02:20
 0:00:59
0:00:59
 0:10:12
0:10:12
 0:04:01
0:04:01
 0:10:31
0:10:31
 0:01:45
0:01:45
 0:02:21
0:02:21
 0:06:06
0:06:06
 0:05:57
0:05:57
 0:10:59
0:10:59
 0:00:58
0:00:58
 0:05:14
0:05:14
 0:05:00
0:05:00
 0:07:36
0:07:36
 0:15:48
0:15:48
 0:07:44
0:07:44
 0:00:56
0:00:56
 0:20:59
0:20:59
 0:04:06
0:04:06
 0:00:53
0:00:53
 0:00:30
0:00:30
 0:09:37
0:09:37