filmov
tv
Solubility vs Concentration - Basic Introduction, Saturated Unsaturated and Supersaturated Solutions

Показать описание
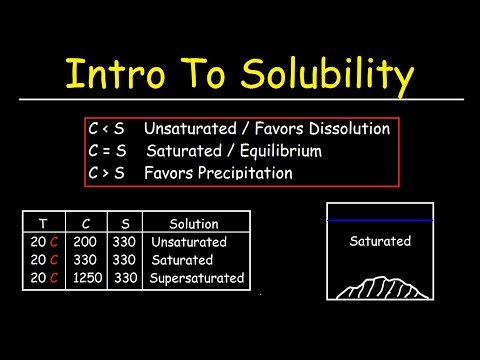
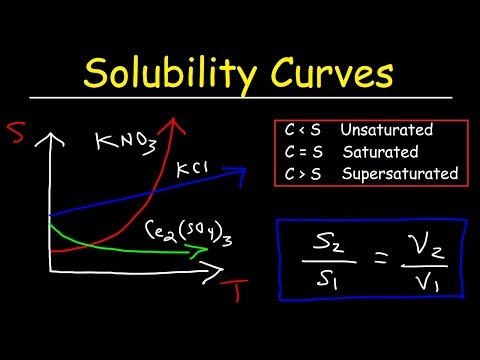
This chemistry video tutorial provides a basic introduction into solubility. It explains the difference between concentration and solubility. Solubility represents the maximum concentration that you can achieve by dissolving a substance in a solution at a given temperature. The solubility of a solution can be increased by increasing the temperature in the case of most ionic compounds. This tutorial discusses the difference between saturated, unsaturated and supersaturated solutions and contains examples & practice problems to illustrate the difference. Saturated solutions are in a state of equilibrium with the solid phase. Unsaturated solutions favor dissolution and supersaturated solutions favor precipitation.
Concentration Formula Sheet:
_____________________________
Heating Curve of Water:
Heating Curve Chemistry Problems:
Final Temperature - Ice Water Mixture:
Molarity, Molality, Density, & Mass %:
Normality & Equivalent Weight:
_________________________________
PPM and PPB Concentrations:
How To Convert PPM to Molarity:
Enthalpy of Solution & Hydration:
Solubility Vs Concentration:
Solubility Curves:
___________________________________
Henry's Law & Gas Solubility:
Vapor Pressure & Clausius Equation:
Raoult's Law - Vapor Pressure:
Colligative Properties:
Chemical Kinetics Initial Rate Method:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Concentration Formula Sheet:
_____________________________
Heating Curve of Water:
Heating Curve Chemistry Problems:
Final Temperature - Ice Water Mixture:
Molarity, Molality, Density, & Mass %:
Normality & Equivalent Weight:
_________________________________
PPM and PPB Concentrations:
How To Convert PPM to Molarity:
Enthalpy of Solution & Hydration:
Solubility Vs Concentration:
Solubility Curves:
___________________________________
Henry's Law & Gas Solubility:
Vapor Pressure & Clausius Equation:
Raoult's Law - Vapor Pressure:
Colligative Properties:
Chemical Kinetics Initial Rate Method:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Комментарии
 0:13:20
0:13:20
 0:03:28
0:03:28
 0:04:29
0:04:29
 0:07:30
0:07:30
 0:06:19
0:06:19
 0:20:37
0:20:37
 0:04:23
0:04:23
 0:03:20
0:03:20
 4:52:15
4:52:15
 0:16:09
0:16:09
 0:05:50
0:05:50
 0:10:36
0:10:36
 0:08:36
0:08:36
 0:10:56
0:10:56
 0:03:36
0:03:36
 0:20:47
0:20:47
 0:07:59
0:07:59
 0:08:46
0:08:46
 0:04:11
0:04:11
 0:09:13
0:09:13
 0:12:16
0:12:16
 0:10:35
0:10:35
 0:41:52
0:41:52
 0:03:01
0:03:01