filmov
tv
Solubility Product Constant (Ksp)
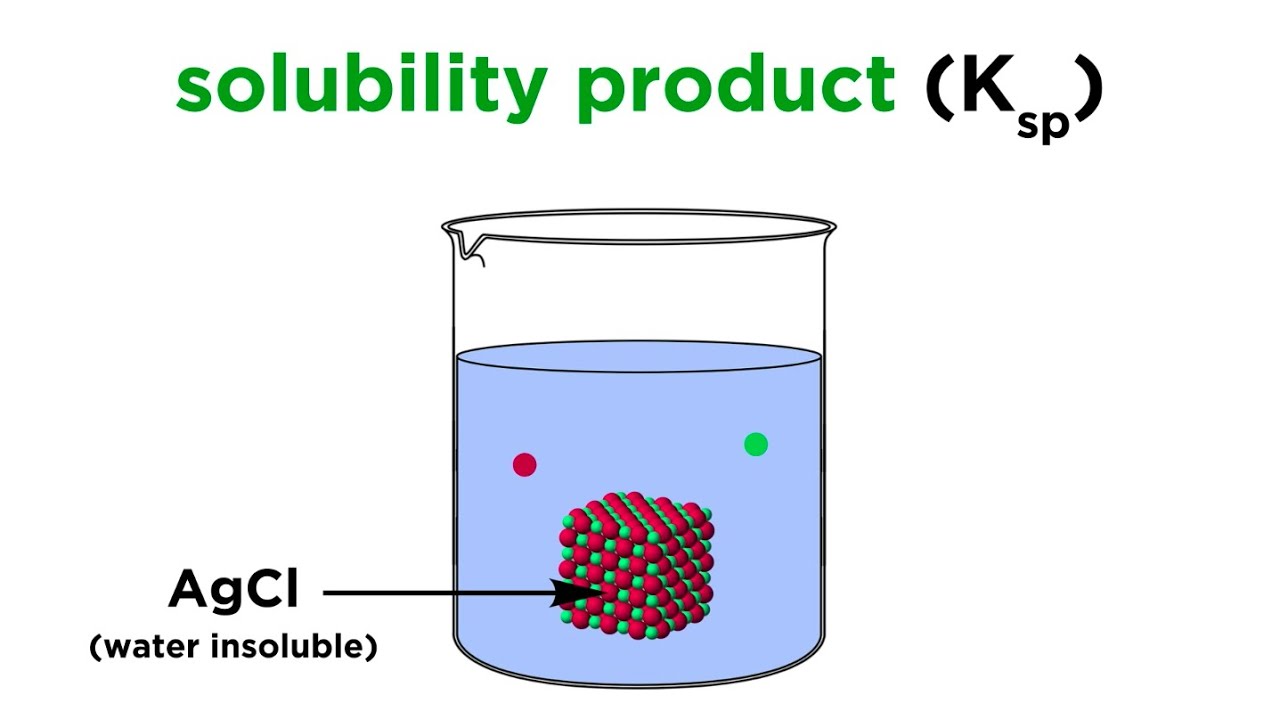
Показать описание
We've learned that some ionic solids are totally water insoluble, but in fact this is a slight oversimplification. Even such solids will dissolve to some minuscule degree, and we can measure this, and do calculations with it. Let's learn about solubility product constants!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Solubility Product Constant (Ksp)
Ksp - Molar Solubility, Ice Tables, & Common Ion Effect
17.4 Solubility and Ksp | General Chemistry
Worked example: Calculating solubility from Kₛₚ | Equilibrium | AP Chemistry | Khan Academy
Ksp Solubility Constant vs Ion Product (Saturation, Unsaturation, Equilibrium) Chemistry
Introduction to solubility equilibria | Equilibrium | AP Chemistry | Khan Academy
solubility product constant ksp for slightly soluble salts
Calculating Ksp
The Solubility Product Constant (Ksp) and Precipitation
Solubility
Calculate Solubility Product Constant (Ksp) From Mass and Volume 002
The Common Ion Effect
The Solubility Product Constant, Ksp
Solubility Equilibrium Practice Problems
Ksp Solubility Product Overview
Practice Problem: Solubility Product Constant Calculations
Solubility Equilibria and the Solubility Product Constant (Ksp)
The Solubility Product Constant (Ksp) and Relative Solubility
Solubility Product Constant Ksp
ALEKS: Writing a solubility product (Ksp) expression
Calculating Molar Solubility Given Solubility Product Constant Ksp | Year 12 HSC Chemistry Module 5
Solubility 2: The Solubility Product Constant, Ksp
Molar Solubility, Solubility Product Constant, and Predicting Precipitation
SCH 4U - 7.6 Solubility Equilibria and Solubility Product Constant (Ksp)
Комментарии
 0:08:36
0:08:36
 0:41:52
0:41:52
 0:22:41
0:22:41
 0:04:52
0:04:52
 0:08:54
0:08:54
 0:08:17
0:08:17
 0:11:01
0:11:01
 0:04:53
0:04:53
 0:13:19
0:13:19
 0:07:06
0:07:06
 0:08:44
0:08:44
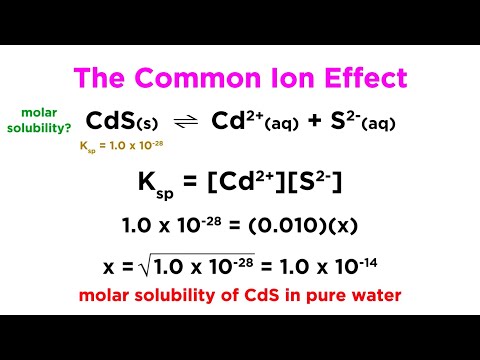 0:04:26
0:04:26
 0:12:55
0:12:55
 0:24:30
0:24:30
 0:08:09
0:08:09
 0:03:03
0:03:03
 0:08:12
0:08:12
 0:05:01
0:05:01
 0:08:14
0:08:14
 0:07:36
0:07:36
 0:06:42
0:06:42
 0:13:39
0:13:39
 0:09:31
0:09:31
 0:27:39
0:27:39