filmov
tv
Solubility Curves - Basic Introduction - Chemistry Problems
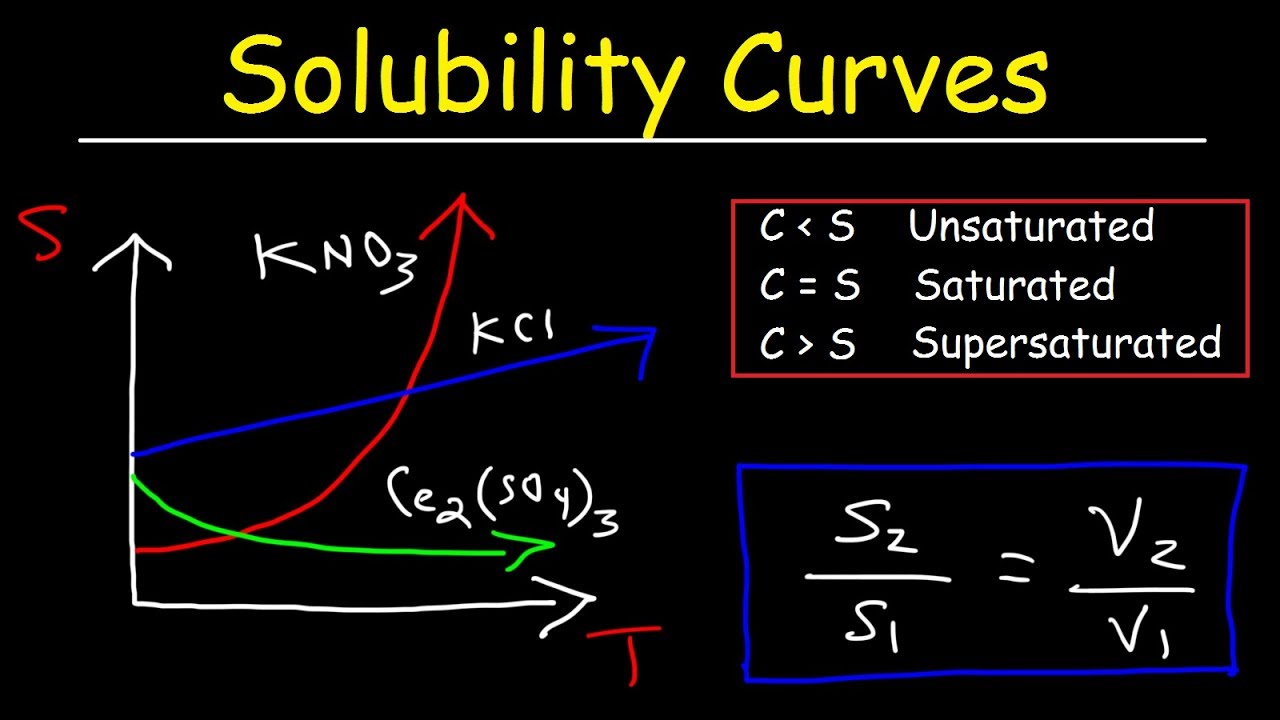
Показать описание
This chemistry video tutorial provides a basic introduction into solubility curves. It contains plenty of examples and practice problems on calculating the solubility of an ionic compound at a given temperature and volume. It explains how to determine the temperature at which two substances have the same solubility in addition to determining if a solution is saturated, unsaturated, or supersaturated under certain conditions.
________________________________
Heating Curve of Water:
Heating Curve Chemistry Problems:
Final Temperature - Ice Water Mixture:
Molarity, Molality, Density, & Mass %:
Normality & Equivalent Weight:
_________________________________
PPM and PPB Concentrations:
How To Convert PPM to Molarity:
Enthalpy of Solution & Hydration:
Solubility Vs Concentration:
Solubility Curves:
___________________________________
Henry's Law & Gas Solubility:
Vapor Pressure & Clausius Equation:
Raoult's Law - Vapor Pressure:
Colligative Properties:
Chemical Kinetics Initial Rate Method:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
________________________________
Heating Curve of Water:
Heating Curve Chemistry Problems:
Final Temperature - Ice Water Mixture:
Molarity, Molality, Density, & Mass %:
Normality & Equivalent Weight:
_________________________________
PPM and PPB Concentrations:
How To Convert PPM to Molarity:
Enthalpy of Solution & Hydration:
Solubility Vs Concentration:
Solubility Curves:
___________________________________
Henry's Law & Gas Solubility:
Vapor Pressure & Clausius Equation:
Raoult's Law - Vapor Pressure:
Colligative Properties:
Chemical Kinetics Initial Rate Method:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Комментарии
 0:20:47
0:20:47
 0:20:37
0:20:37
 0:04:24
0:04:24
 0:09:13
0:09:13
 0:13:20
0:13:20
 0:03:01
0:03:01
 0:04:29
0:04:29
 0:15:10
0:15:10
 0:08:57
0:08:57
 0:23:22
0:23:22
 0:09:13
0:09:13
 0:03:07
0:03:07
 0:09:23
0:09:23
 0:12:43
0:12:43
 0:09:23
0:09:23
 0:01:09
0:01:09
 0:00:43
0:00:43
 0:03:32
0:03:32
 0:14:14
0:14:14
 0:05:02
0:05:02
 0:03:56
0:03:56
 0:00:40
0:00:40
 0:06:59
0:06:59
 0:10:14
0:10:14