filmov
tv
Structure and stability of common carbocations

Показать описание
00:21 Fluoromethane (methyl fluoride)
00:50 Chloromethane (methyl chloride)
01:11 Bromomethane (methyl bromide)
01:29 Iodomethane (methyl iodide)
02:01 Polarity of halo-carbon bond
02:42 Methyl carbocation
03:29 Table
04:29 Ethyl carbocation
06:25 Ethyl carbocation (side view), showing hyperconjugation interaction
08:22 1-propyl (n-propyl) carbocation
10:37 2-propyl (iso-propyl) carbocation
11:45 tert-butyl (2-methyl-2-propyl) carbocation
Computed enthalpies of formation of carbocations from haloalkanes, and the relative stability, are demonstrated. Single point energies computed using MP4/6-311++G(2d,p). Figures created using ChemCraft program.
Don't forget to like, comment, share, and subscribe!
00:50 Chloromethane (methyl chloride)
01:11 Bromomethane (methyl bromide)
01:29 Iodomethane (methyl iodide)
02:01 Polarity of halo-carbon bond
02:42 Methyl carbocation
03:29 Table
04:29 Ethyl carbocation
06:25 Ethyl carbocation (side view), showing hyperconjugation interaction
08:22 1-propyl (n-propyl) carbocation
10:37 2-propyl (iso-propyl) carbocation
11:45 tert-butyl (2-methyl-2-propyl) carbocation
Computed enthalpies of formation of carbocations from haloalkanes, and the relative stability, are demonstrated. Single point energies computed using MP4/6-311++G(2d,p). Figures created using ChemCraft program.
Don't forget to like, comment, share, and subscribe!
Structure and stability of common carbocations
Resonance Made Easy! Finding the Most Stable Resonance Structure - Organic Chemistry
Type Of Supports Steel Column to Beam Connections #construction #civilengineering #engineering
The Real Reason Buildings Fall #shorts #civilengineering #construction #column #building #concrete
Modules for Learning Structural Stability
Constipation - 3d animation #meded #anatomy #3dmodel
Reinforcing Slopes:The Process of Shotcrete Application
Stiffeners in Columns | Importance & Usage in Structural Design
Is Reality Your Brain's Creation? Jacobo Grinberg's Syntergic Theory 🤯
Straight Spine Posture: How to fix your pelvis rotation
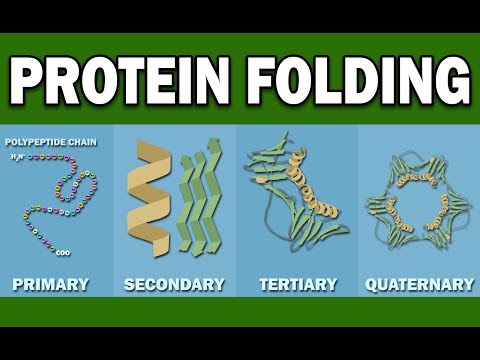
PROTEIN FOLDING
Protein Structure
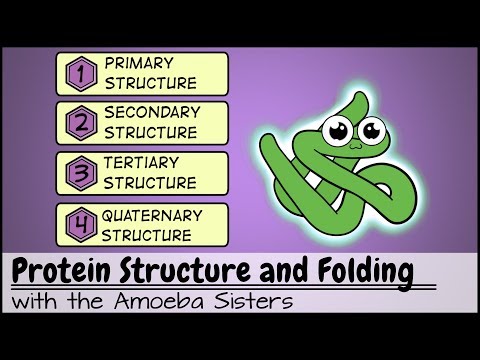
Protein Structure and Folding
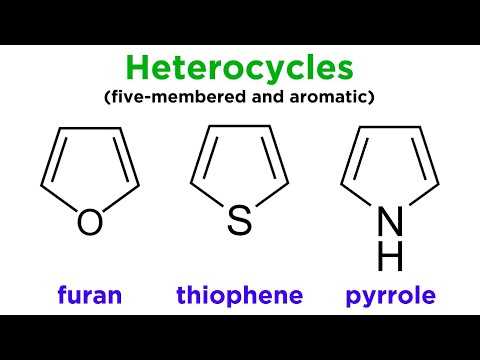
Heterocycles Part 1: Furan, Thiophene, and Pyrrole
Exceptions To The Octet Rule - Lewis Dot Diagrams
Understanding and Analysing Trusses
Torsion in Beams – Causes & Remedies
3 ACL tears 🦵#acl #acltear #aclsurgeryrecovery #kneeinjury
Construction Practices: Flat slab
Structural Comparisons of Acidity and Basicity; The Stability Factors
Amazing Idea 👏🏻 #Earthquake Proof Building #testingvideo #earthquake #turkeyearthquake
Cyclohexane - Basics, Structure, & Stability | Organic Chemistry | One Chemistry
Healthier feet are just 4 exercises away!
How atoms bond - George Zaidan and Charles Morton
Комментарии
 0:13:13
0:13:13
 0:08:25
0:08:25
 0:00:06
0:00:06
 0:00:05
0:00:05
 1:34:26
1:34:26
 0:00:21
0:00:21
 0:00:06
0:00:06
 0:00:05
0:00:05
 1:56:13
1:56:13
 0:00:17
0:00:17
 0:04:32
0:04:32
 0:10:50
0:10:50
 0:07:46
0:07:46
 0:07:30
0:07:30
 0:12:35
0:12:35
 0:17:41
0:17:41
 0:00:19
0:00:19
 0:00:22
0:00:22
 0:00:09
0:00:09
 0:08:21
0:08:21
 0:00:05
0:00:05
 0:14:30
0:14:30
 0:00:15
0:00:15
 0:03:34
0:03:34