filmov
tv
Exceptions To The Octet Rule - Lewis Dot Diagrams

Показать описание
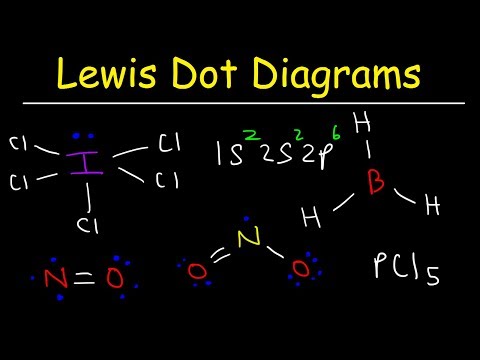
This chemistry video tutorial discusses the exceptions to the octet rule while providing the lewis dot diagrams of the molecular compounds involved. BH3 has an incomplete octet - that is, it has less than 8 electrons. Molecules such as ICl5 and PCl5 have an expanded octet which means the center atom has more than 8 electrons. The last category are molecules with odd number of electrons such as NO and NO2. These will always be electron deficient and contain an incomplete octet at the nitrogen atom.
Molecular Geometry - Free Formula Sheet:
Chemistry 1 Final Exam Review:
_____________________________
How To Draw Lewis Structures:
VSEPR Theory:
Molecular Geometry:
Lewis Dot Structures:
Lewis Structures of Ionic Compounds:
_________________________________
Octet Rule Exceptions:
Resonance Structures:
Polar and Nonpolar Molecules:
Formal Charge Calculations:
Lewis Structures - Mega Review:
________________________________
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Dipole Dipole Forces of Attraction:
Hydrogen Bonding:
Unit Cell Chemistry:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Molecular Geometry - Free Formula Sheet:
Chemistry 1 Final Exam Review:
_____________________________
How To Draw Lewis Structures:
VSEPR Theory:
Molecular Geometry:
Lewis Dot Structures:
Lewis Structures of Ionic Compounds:
_________________________________
Octet Rule Exceptions:
Resonance Structures:
Polar and Nonpolar Molecules:
Formal Charge Calculations:
Lewis Structures - Mega Review:
________________________________
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Dipole Dipole Forces of Attraction:
Hydrogen Bonding:
Unit Cell Chemistry:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:12:35
0:12:35
 0:07:10
0:07:10
 0:04:31
0:04:31
 0:04:22
0:04:22
 0:07:36
0:07:36
 0:06:28
0:06:28
 0:15:12
0:15:12
 0:06:09
0:06:09
 0:05:13
0:05:13
 0:16:30
0:16:30
 0:09:22
0:09:22
 0:12:15
0:12:15
 0:36:27
0:36:27
 0:05:22
0:05:22
 0:01:24
0:01:24
 0:04:21
0:04:21
 0:00:11
0:00:11
 0:16:06
0:16:06
 0:05:28
0:05:28
 0:10:48
0:10:48
 0:07:47
0:07:47
 0:13:22
0:13:22
 0:04:41
0:04:41
 0:18:38
0:18:38