filmov
tv
How to Write the Orbital Diagram for Lithium (Li)

Показать описание
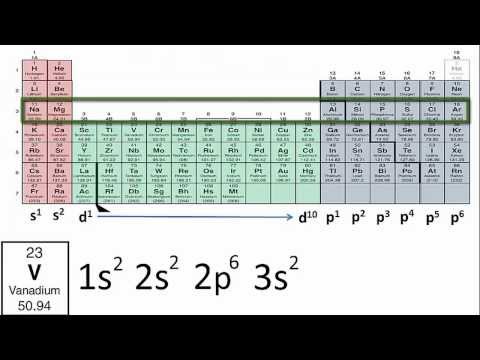
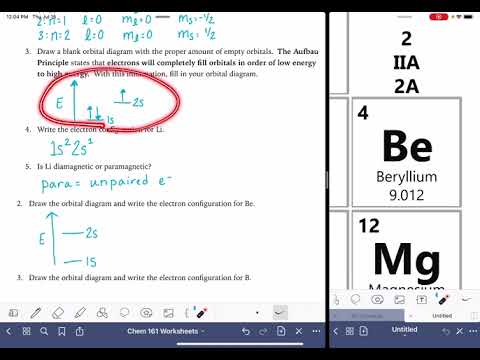
To write the orbital diagram for the Lithium atom (Li) first we need to write the electron configuration for just Li. To do that we need to find the number of electrons for the Li atom (there are 3 electrons) using the Periodic Table. When we write the configuration, we'll put all 3 electrons in orbitals around the nucleus of the Lithium atom.
Either way, the Lithium electron configuration will be 1s2 2s1 .
Once we have this information we can then place electrons in an orbital diagram for Lithium. Note that we are making the Atomic Orbital Diagram for Li (not a molecular orbital diagram).
The configuration notation and atomic orbital diagram provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
Either way, the Lithium electron configuration will be 1s2 2s1 .
Once we have this information we can then place electrons in an orbital diagram for Lithium. Note that we are making the Atomic Orbital Diagram for Li (not a molecular orbital diagram).
The configuration notation and atomic orbital diagram provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
Orbital Diagrams and Electron Configuration - Basic Introduction - Chemistry Practice Problems
Electron Configuration - Basic introduction
How to Write Electron Configurations and Orbital Diagrams (General Chemistry I)
How to Write the Electron Configuration for an Element in Each Block
How to Write Orbital Diagrams
Writing Electron Configurations Using Only the Periodic Table
How to Write Orbital Filling Diagrams for Atoms: Examples & Practice
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Condensed Matter Physics - Diamagnetism : Classical Theory of Diamagnetism
How to Draw Orbital Diagrams and Hund's Rule | Study Chemistry With Us
chemistry #orbital diagrams of atoms of the 1st 20 elements.
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers
How to Write the Orbital Diagram for Oxygen (O)
Aufbau's Principle, Hund's Rule & Pauli's Exclusion Principle - Electron Configur...
Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory
How to Draw an Orbital Diagram EASIEST TUTORIAL #chemistry #shorts #science #education #homework
How to Write Electron Configuration 6 examples + Exceptions Chromium & Copper (Chemistry)
How to Write the Orbital Diagram for Lithium (Li)
Orbital diagrams
Orbital Diagrams | How to Write Orbital Diagrams | Orbital Diagram Shapes
How to Write the Atomic Orbital Diagram for Selenium (Se)
Molecular Orbital Diagram 👍👍✍️✍️
Orbit vs Orbital #class11 #chemistry #pw #atomicstructure
36a: Writing electron configurations and orbital diagrams
Комментарии
 0:12:12
0:12:12
 0:10:19
0:10:19
 0:16:01
0:16:01
 0:07:23
0:07:23
 0:02:43
0:02:43
 0:04:52
0:04:52
 0:07:07
0:07:07
 0:08:42
0:08:42
 0:32:33
0:32:33
 0:05:37
0:05:37
 0:00:08
0:00:08
 0:11:19
0:11:19
 0:01:53
0:01:53
 0:05:24
0:05:24
 0:07:54
0:07:54
 0:01:01
0:01:01
 0:04:32
0:04:32
 0:01:35
0:01:35
 0:07:29
0:07:29
 0:09:00
0:09:00
 0:02:15
0:02:15
 0:00:15
0:00:15
 0:00:08
0:00:08
 0:15:10
0:15:10