filmov
tv
How to Write Orbital Diagrams

Показать описание
How to create orbital diagrams using electron configurations.
TRANSCRIPT:
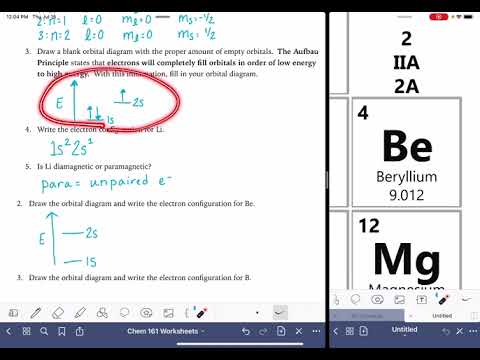
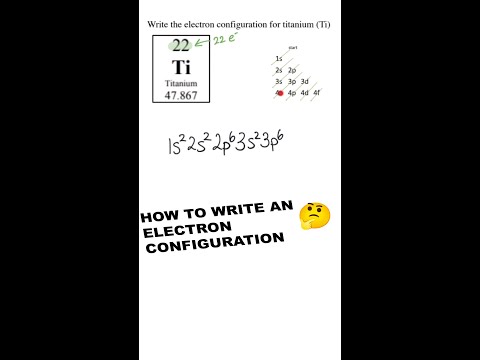
So here I’m given the electron configurations of a couple different elements, and what I want to do is draw an orbital diagram for both of them. What you’re supposed to do is write what’s here, so basically you’re going to write each of these that I’ve underlined (which is the shell) and then you write a line next to it. So I have 1s and I’m going to put a line there. Sometimes people put boxes. You can do either one of them. And then what you’re supposed to do is represent each electron with a half up arrow and a half down arrow. Since there’s only 1 electron in hydrogen, we’re just going to put one half up arrow. And that’s basically it – that’s the orbital diagram for hydrogen. Now onto something a little harder, we have nitrogen. We have 1s, 2s, and then, for 2p, you have to remember that the p block can contain 6 electrons as you can see from this periodic table. So 1, 2, 3, 4, 5, 6. And if you’re wondering why this is all marked up, it’s because I was writing on it for my previous video. So since we have 6 electrons, we can have 2 electrons on each line. So 1, 2, 3 because 6 divided by 2 is 3. Now we have 1s2, so 1 electron is going to be represented by a half up arrow, the other electron is going to be represented by a half down arrow. And this is because when orbitals are in the same sublevel, they have to have different spins. And this is called Pauli’s rule. It’s going to be the same thing for 2s, then for 2p, we have 3 electrons in the 2p sublevel. But we can’t just go like this because that’s actually not allowed. This is called Hund’s rule where we have to go and fill each of the lines before putting two of them together on the same line. And that’s because it’s the lowest energy so it’s the most favorable. So we have each of these electrons in their own sublevel, instead of this.
TRANSCRIPT:
So here I’m given the electron configurations of a couple different elements, and what I want to do is draw an orbital diagram for both of them. What you’re supposed to do is write what’s here, so basically you’re going to write each of these that I’ve underlined (which is the shell) and then you write a line next to it. So I have 1s and I’m going to put a line there. Sometimes people put boxes. You can do either one of them. And then what you’re supposed to do is represent each electron with a half up arrow and a half down arrow. Since there’s only 1 electron in hydrogen, we’re just going to put one half up arrow. And that’s basically it – that’s the orbital diagram for hydrogen. Now onto something a little harder, we have nitrogen. We have 1s, 2s, and then, for 2p, you have to remember that the p block can contain 6 electrons as you can see from this periodic table. So 1, 2, 3, 4, 5, 6. And if you’re wondering why this is all marked up, it’s because I was writing on it for my previous video. So since we have 6 electrons, we can have 2 electrons on each line. So 1, 2, 3 because 6 divided by 2 is 3. Now we have 1s2, so 1 electron is going to be represented by a half up arrow, the other electron is going to be represented by a half down arrow. And this is because when orbitals are in the same sublevel, they have to have different spins. And this is called Pauli’s rule. It’s going to be the same thing for 2s, then for 2p, we have 3 electrons in the 2p sublevel. But we can’t just go like this because that’s actually not allowed. This is called Hund’s rule where we have to go and fill each of the lines before putting two of them together on the same line. And that’s because it’s the lowest energy so it’s the most favorable. So we have each of these electrons in their own sublevel, instead of this.
Комментарии
 0:12:12
0:12:12
 0:02:43
0:02:43
 0:05:37
0:05:37
 0:16:01
0:16:01
 0:04:28
0:04:28
 0:07:07
0:07:07
 0:07:29
0:07:29
 0:01:01
0:01:01
 0:09:00
0:09:00
 0:07:23
0:07:23
 0:00:08
0:00:08
 0:01:53
0:01:53
 0:15:10
0:15:10
 0:05:24
0:05:24
 0:03:13
0:03:13
 0:04:08
0:04:08
 0:08:08
0:08:08
 0:11:05
0:11:05
 0:08:50
0:08:50
 0:09:05
0:09:05
 0:10:19
0:10:19
 0:08:42
0:08:42
 0:01:00
0:01:00
 0:06:02
0:06:02