filmov
tv
How to Write Electron Configurations and Orbital Diagrams (General Chemistry I)

Показать описание
*I recommend watching this in x1.25 - 1.5 speed
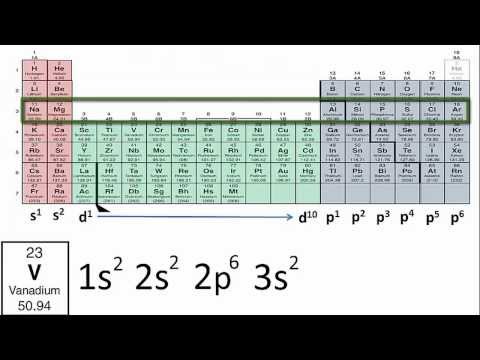
This video goes over how to write out electron configurations and electron orbital diagrams, as well as explains some useful techniques to make these problems much easier. I think the most important step to understanding these is equating the s, p, d, and f orbitals to the corresponding areas of the period table. That way you'll always be sure of how many electrons and boxes are in each orbital, and from there its as simple as counting up to the correct number of electrons.
This video tutorial was requested in a comment on a previous video. Hope this helps you Artour! Up next I'll upload another requested video from a comment- this time on identifying oxidation/reduction in chemical equations. Let me know below in the comments if anyone would like more examples of any particular problem type. What are you guys struggling with most?
Hope this helps someone! Let me know down below if:
- you have an easier way to do these
- you found a mistake or want clarification on something
- you found this helpful :D
* I am not an expert in this topic. I am just a clinical lab scientist and life-long student who learns best from videos/visual representations and demonstration and have often turned to Youtube for help learning. My hope is that others in the same boat find and benefit from this.
Main Helpful Sources:
-Khan Academy
This video goes over how to write out electron configurations and electron orbital diagrams, as well as explains some useful techniques to make these problems much easier. I think the most important step to understanding these is equating the s, p, d, and f orbitals to the corresponding areas of the period table. That way you'll always be sure of how many electrons and boxes are in each orbital, and from there its as simple as counting up to the correct number of electrons.
This video tutorial was requested in a comment on a previous video. Hope this helps you Artour! Up next I'll upload another requested video from a comment- this time on identifying oxidation/reduction in chemical equations. Let me know below in the comments if anyone would like more examples of any particular problem type. What are you guys struggling with most?
Hope this helps someone! Let me know down below if:
- you have an easier way to do these
- you found a mistake or want clarification on something
- you found this helpful :D
* I am not an expert in this topic. I am just a clinical lab scientist and life-long student who learns best from videos/visual representations and demonstration and have often turned to Youtube for help learning. My hope is that others in the same boat find and benefit from this.
Main Helpful Sources:
-Khan Academy
Комментарии
 0:10:19
0:10:19
 0:07:23
0:07:23
 0:04:52
0:04:52
 0:10:17
0:10:17
 0:08:42
0:08:42
 0:07:54
0:07:54
 0:04:36
0:04:36
 0:12:12
0:12:12
 0:45:49
0:45:49
 0:04:59
0:04:59
 0:03:31
0:03:31
 0:03:53
0:03:53
 0:17:24
0:17:24
 0:06:29
0:06:29
 0:30:45
0:30:45
 0:10:06
0:10:06
 0:16:06
0:16:06
 0:16:01
0:16:01
 0:05:07
0:05:07
 0:12:57
0:12:57
 0:12:13
0:12:13
 0:04:14
0:04:14
 0:04:43
0:04:43
 0:03:45
0:03:45