filmov
tv
Ionic Bonds, Polar Covalent Bonds, and Nonpolar Covalent Bonds
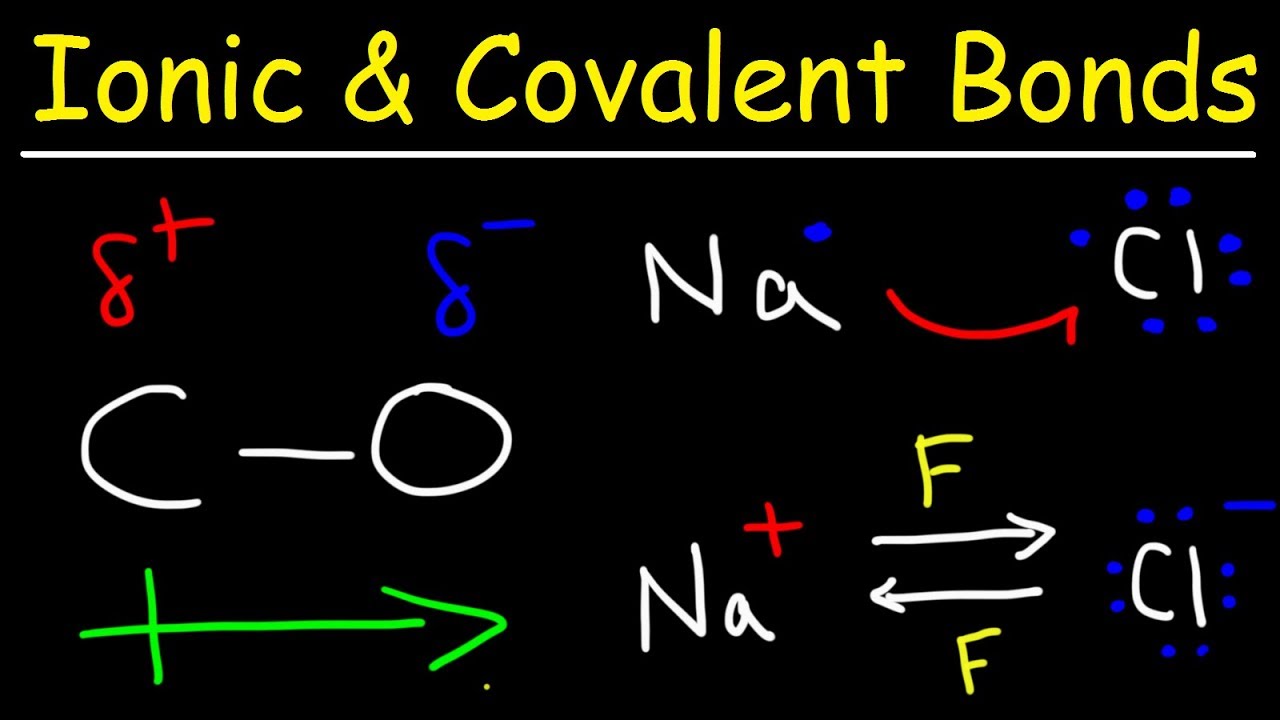
Показать описание
This organic chemistry video tutorial explains how to identify a bond as an ionic bond, polar covalent bond, or a nonpolar covalent bond. Ionic bonds usually consist of metals and nonmetals where as covalent bonds consists of nonmetals. In a nonpolar covalent bond, electrons are shared equally and the electronegativity difference between the two atoms is 0.4 or less. For polar covalent bonds, the electrons are shared unequally between the two atoms and the electronegativity difference is defined to be 0.5 or more.
Organic Chemistry - Basic Introduction:
Which Bond Is More Polar?
How To Draw Lewis Structures:
Condensed Structures to Skeletal Structures:
Functional Groups Review:
Primary, Secondary, & Tertiary Functional Groups:
_________________________________
How To Calculate Formal Charge:
Finding Lone Pairs Using Formal Charge:
Dipole Moment & Electronegativity:
Predicting Bond Angles:
Valence Bond Theory:
Hybridization of Atomic Orbitals:
_______________________________
Bond Strength and Bond Length:
Orbital Overlap and Bond Length:
Organic Chemistry PDF Worksheets:
Organic Chemistry Exam 1 Playlist:
Full-Length Videos and Worksheets:
Organic Chemistry - Basic Introduction:
Which Bond Is More Polar?
How To Draw Lewis Structures:
Condensed Structures to Skeletal Structures:
Functional Groups Review:
Primary, Secondary, & Tertiary Functional Groups:
_________________________________
How To Calculate Formal Charge:
Finding Lone Pairs Using Formal Charge:
Dipole Moment & Electronegativity:
Predicting Bond Angles:
Valence Bond Theory:
Hybridization of Atomic Orbitals:
_______________________________
Bond Strength and Bond Length:
Orbital Overlap and Bond Length:
Organic Chemistry PDF Worksheets:
Organic Chemistry Exam 1 Playlist:
Full-Length Videos and Worksheets:
Комментарии
 0:11:00
0:11:00
 0:03:33
0:03:33
 0:04:55
0:04:55
 0:12:14
0:12:14
 0:04:30
0:04:30
 0:07:23
0:07:23
 0:07:11
0:07:11
 0:00:57
0:00:57
 0:46:39
0:46:39
 0:13:48
0:13:48
 0:09:46
0:09:46
 0:04:58
0:04:58
 0:02:09
0:02:09
 0:02:15
0:02:15
 0:01:23
0:01:23
 0:06:30
0:06:30
 0:03:40
0:03:40
 0:11:20
0:11:20
 0:15:27
0:15:27
 0:09:16
0:09:16
 0:21:57
0:21:57
 0:07:20
0:07:20
 0:00:15
0:00:15
 0:01:00
0:01:00