filmov
tv
Covalent bonds | Molecular and ionic compound structure and properties | AP Chemistry | Khan Academy
Показать описание
Covalent bonds involve the sharing of electron pairs between atoms. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent bond (e.g., H–H or C–H), while electrons shared between atoms of unequal electronegativity constitute a polar covalent bond (e.g., H–O).
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
GCSE Chemistry - Covalent Bonding #16
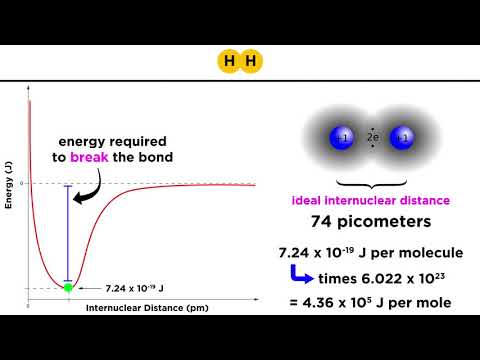
Covalent bonds | Molecular and ionic compound structure and properties | AP Chemistry | Khan Academy
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar
GCSE Chemistry - Properties of Simple Molecular Substances & Giant Covalent Structures #17
What Are Covalent Bonds | Properties of Matter | Chemistry | FuseSchool
Naming Covalent Molecular Compounds
How To Name Covalent Molecular Compounds - The Easy Way!
Chemical Bonding - Ionic vs. Covalent Bonds
Chemical Bonding - 1 - Introduction | Ionic Bond | Covalent Bond | Fajan's Rules
Covalent Bond Energy and Length
Atomic Hook-Ups - Types of Chemical Bonds: Crash Course Chemistry #22
How atoms bond - George Zaidan and Charles Morton
Covalent Bonding | #aumsum #kids #science #education #children
Covalent Bonding - Dot and Cross Diagrams - p86
What are Covalent Bonds? | Don't Memorise
Why do atoms form molecules? The quantum physics of chemical bonds explained
Chemical Bonds: Ionic and Covalent
Chemical Bonding Explained | Ionic, Covalent and Metallic | GCSE Chemistry
Ionic/Covalent/Metallic Bonds Simply Explained
Ionic Bonds, Polar Covalent Bonds, and Nonpolar Covalent Bonds
Bonding Models and Lewis Structures: Crash Course Chemistry #24
Ionic and Covalent Bonding - Chemistry
Types of Bonding (Ionic, Covalent, Metallic) - GCSE Chemistry Revision
INTRAMOLECULAR BONDING - COVALENT, IONIC, METALLIC
Комментарии
 0:05:33
0:05:33
 0:05:43
0:05:43
 0:03:33
0:03:33
 0:04:46
0:04:46
 0:05:53
0:05:53
 0:10:46
0:10:46
 0:10:11
0:10:11
 0:02:15
0:02:15
 0:43:33
0:43:33
 0:05:47
0:05:47
 0:09:46
0:09:46
 0:03:34
0:03:34
 0:05:30
0:05:30
 0:04:45
0:04:45
 0:05:48
0:05:48
 0:13:25
0:13:25
 0:04:30
0:04:30
 0:03:03
0:03:03
 0:04:40
0:04:40
 0:11:00
0:11:00
 0:11:38
0:11:38
 0:21:57
0:21:57
 0:11:50
0:11:50
 0:06:07
0:06:07