filmov
tv
Calculate the Theoretical Yield to determine the % yield in a chemical reaction

Показать описание
Determine the Theoretical yield (the maximum amount of product that can be produced when 2 values for 2 reactants are given). Then determine the % yield, assuming the actual yield (the amount of product actually weighed out when the reaction is carried out in the lab) is given.
How To Calculate Theoretical Yield and Percent Yield
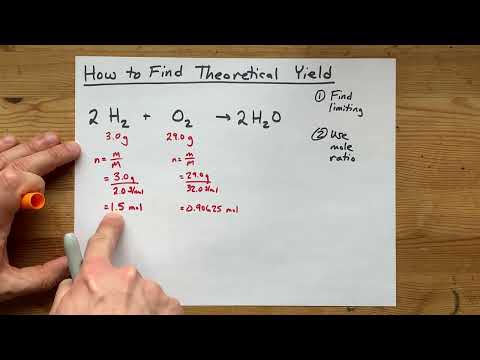
How to Find Theoretical Yield (2023)
How to Calculate Theoretical Yields
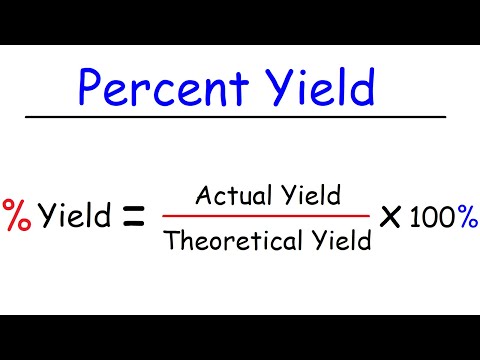
How To Calculate Theoretical Yield and Percent Yield
Calculate the Theoretical Yield to determine the % yield in a chemical reaction
Limiting Reagents and Percent Yield
How to Find Actual Yield, Theoretical Yield, and Percent Yield Examples, Practice Problems
How To Calculate The Percent Yield and Theoretical Yield
Stoichiometry - Part 5
GCSE Chemistry - Percentage Yield #33
Theoretical Yield & Losses | Chemical Calculations | Chemistry | FuseSchool
Theoretical Yield & Percent Yield in Chemistry Explained
Percent Yield Made Easy: Stoichiometry Tutorial Part 4
Theoretical yield: How to calculate PART 1 | Quantitative aspects of chemical change
How to Calculate Percent Yield and Theoretical Yield The Best Way - TUTOR HOTLINE
How to calculate Theoretical Yield and Percent Yield?
Ch#1|Lec#4|Theoretical Yield Actual Yield And Percentage Yield, numericals
Calculating Theoretical & % Yield
How to calculate theoretical yield [ORGANIC CHEMISTRY LAB]
Percentage yield calculation
How to Calculate Theoretical Yield and Percent Yield - Topics in General Chemistry
Quantitative Aspects of Chemical Change: Percentage Yield
Percentage yield calculation | how to calculate % yield
Lab 4 Theoretical Yield Aspirin Calculations
Комментарии
 0:06:24
0:06:24
 0:05:22
0:05:22
 0:04:15
0:04:15
 0:11:03
0:11:03
 0:07:22
0:07:22
 0:04:35
0:04:35
 0:06:17
0:06:17
 0:17:02
0:17:02
 0:29:25
0:29:25
 0:04:54
0:04:54
 0:04:10
0:04:10
 0:23:46
0:23:46
 0:07:45
0:07:45
 0:12:45
0:12:45
 0:21:30
0:21:30
 0:10:40
0:10:40
 0:10:43
0:10:43
 0:14:02
0:14:02
 0:05:29
0:05:29
 0:07:29
0:07:29
 0:07:32
0:07:32
 0:11:52
0:11:52
 0:08:25
0:08:25
 0:02:13
0:02:13