filmov
tv
Ionic Radius Potassium Periodic Trend

Показать описание
Get your free Ultimate Chemistry Cheat Sheet here:
Ionic Radius Potassium Periodic Trend
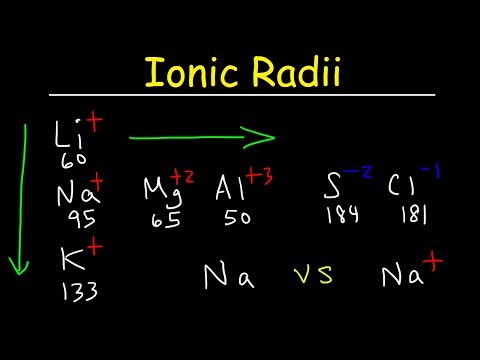
The ionic radius of potassium is smaller than the atomic radius of potassium because it has one less electron than its atom. With one less electron, there is less repulsion among the outermost electrons, causing its radius to decrease. This is the periodic trend in ionic radii with cations, also known as positive ions.
Today's chemistry question of the day:
Which of the following has the smallest radius?
A. K+
B. K
ionic radius periodic trend
ionic radius potassium
ionic radius k+
Ionic Radius Potassium Periodic Trend
The ionic radius of potassium is smaller than the atomic radius of potassium because it has one less electron than its atom. With one less electron, there is less repulsion among the outermost electrons, causing its radius to decrease. This is the periodic trend in ionic radii with cations, also known as positive ions.
Today's chemistry question of the day:
Which of the following has the smallest radius?
A. K+
B. K
ionic radius periodic trend
ionic radius potassium
ionic radius k+
 0:01:27
0:01:27
 0:11:47
0:11:47
 0:09:00
0:09:00
 0:09:40
0:09:40
 0:07:27
0:07:27
 0:04:13
0:04:13
 0:06:32
0:06:32
 0:02:02
0:02:02
 0:14:04
0:14:04
 0:25:57
0:25:57
 0:10:50
0:10:50
 0:11:50
0:11:50
 0:06:09
0:06:09
 0:03:39
0:03:39
 0:17:39
0:17:39
 0:00:31
0:00:31
 0:00:16
0:00:16
 0:13:01
0:13:01
 0:04:48
0:04:48
 0:00:59
0:00:59
 0:14:08
0:14:08
 0:00:58
0:00:58
 0:15:28
0:15:28
 0:15:12
0:15:12