filmov
tv
Ionic Radius | Trends of Ionic Radius in Periodic Table

Показать описание
This lecture is about ionic radius and trends of ionic radius in the periodic table.
Q: What is ionic radius?
.
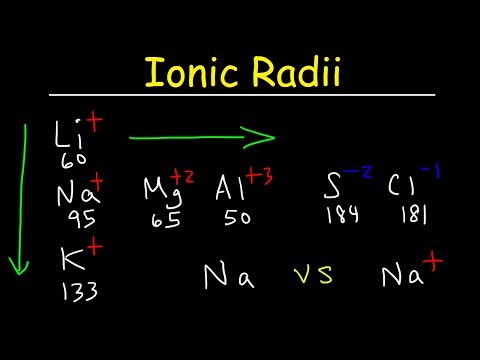
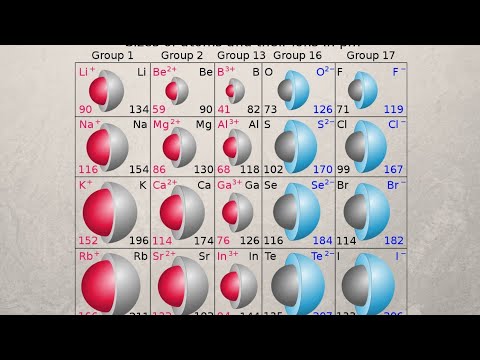
Ans: The distance between the nucleus and outermost valence shell of an ion is called ionic radius.
For example, the atomic radius of sodium is 186 pm and the ionic radius of sodium is 95 pm.
You will also learn the following conceptual questions:
Q: What is atomic radii?
Q: Why the size of cations is smaller than its parent atom?
Q: Why the size of anion is greater than its parent atom?
To learn more about atomic radii, watch this lecture till the end.
#ionicradius
#ionicvsatomicradius
#chemistry
Q: What is ionic radius?
.
Ans: The distance between the nucleus and outermost valence shell of an ion is called ionic radius.
For example, the atomic radius of sodium is 186 pm and the ionic radius of sodium is 95 pm.
You will also learn the following conceptual questions:
Q: What is atomic radii?
Q: Why the size of cations is smaller than its parent atom?
Q: Why the size of anion is greater than its parent atom?
To learn more about atomic radii, watch this lecture till the end.
#ionicradius
#ionicvsatomicradius
#chemistry
Ionic Radius Trends, Basic Introduction, Periodic Table, Sizes of Isoelectric Ions, Chemistry
Ionic Radius | Trends of Ionic Radius in Periodic Table
Ionic Radius Trend of the Periodic Table | Metal and Nonmetal Ionic Radii Trend
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
Practice Problem: Atomic Radii and Ionic Radii
46: Periodic trends: Ionic radius
S3.1.3 Trends in ionic radii
Ionic Radius
9701/42/M/J/24/Q1 Cambridge International A Level Chemistry 9701 May/June 2024 Paper 42 Q1
Ionic Radius Trends - Periodic Table - Chemistry Class 11
Class 12 |Ch#13 |Lec #2 |Ionization energy, Trends in ionic radius#2ndyearchemistry
Periodic Trends: Ionic Radius (Ionic Size) | Study Chemistry With Us
Ionic Radius Trends
Atomic and ionic radii | Periodic table | Chemistry | Khan Academy
IONIC RADIUS#IONIC RADII TRENDS ACROSS THE PERIODIC TABLE
L-8. Ionic radius || Trends of ionic radius in periodic table #IonicRadius
3.2 Trends in ionic radius (SL)
Periodic Trends - Atomic Radius, Electronegativity, Ionization Energy - Chemistry Series
3.2/S3.1.3 Trends in Atomic Radii [SL IB Chemistry]
3.2 Trends in ionic radii (SL)
Ionic Radius | Periodic Table class 11 | IIT JEE/NEET | Poonam mam | ATP STAR KOTA
Periodic Trends Part 1 | Atomic and Ionic Radius
Periodic Trends, Ionic Radius, Cation, Anion - AP Chemistry
What is Atomic Radius? Periodic Trends
Комментарии
 0:11:47
0:11:47
 0:09:00
0:09:00
 0:06:32
0:06:32
 0:07:53
0:07:53
 0:07:27
0:07:27
 0:07:38
0:07:38
 0:06:57
0:06:57
 0:04:40
0:04:40
 0:13:24
0:13:24
 0:05:17
0:05:17
 0:21:51
0:21:51
 0:16:10
0:16:10
 0:07:41
0:07:41
 0:11:26
0:11:26
 0:07:33
0:07:33
 0:24:46
0:24:46
 0:04:53
0:04:53
 0:18:06
0:18:06
 0:03:23
0:03:23
 0:04:29
0:04:29
 0:23:58
0:23:58
 0:07:56
0:07:56
 0:01:40
0:01:40
 0:08:04
0:08:04